Cytotoxicity on human cells of Cry1Ab and Cry1Ac Bt insecticidal toxins alone or with a glyphosate-based herbicideby R. Mesnage,a,b E. Clair,a,b S. Gress,a,b C. Then,c A. Székácsd and G.-E. Séralinia,b*
J. Appl. Toxicol. 2011 Copyright © 2011 John Wiley & Sons, Ltd.
Received: 5 May 2011, Revised: 15 November 2011, Accepted: 19 November 2011 Published online in Wiley Online Library (wileyonlinelibrary.com) DOI 10.1002/jat.2712
UNCORRECTED PROOFNOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHTYOU ARE REQUIRED TO READ
THE COPYRIGHT NOTICE AT THIS LINK BEFORE YOU READ THE FOLLOWING WORK, THAT IS AVAILABLE SOLELY FOR PRIVATE STUDY, SCHOLARSHIP OR RESEARCH PURSUANT TO 17 U.S.C. SECTION 107 AND 108. IN THE EVENT THAT THE LIBRARY DETERMINES THAT UNLAWFUL COPYING OF THIS WORK HAS OCCURRED, THE LIBRARY HAS THE RIGHT TO BLOCK THE I.P. ADDRESS AT WHICH THE UNLAWFUL COPYING APPEARED TO HAVE OCCURRED. THANK YOU FOR RESPECTING THE RIGHTS OF COPYRIGHT OWNERS.
ABSTRACT: The study of combined effects of pesticides represents a challenge for toxicology. In the case of the new growing generation of genetically modified (GM) plants with stacked traits, glyphosate-based herbicides (like Roundup) residues are present in the Roundup-tolerant edible plants (especially corns) and mixed with modified Bt insecticidal toxins that are produced by the GM plants themselves. The potential side effects of these combined pesticides on human cells are investigated in this work. Here we have tested for the very first time Cry1Ab and Cry1Ac Bt toxins (10 ppb to 100 ppm) on the human embryonic kidney cell line 293, as well as their combined actions with Roundup, within 24h, on three biomarkers of cell death: measurements of mitochondrial succinate dehydrogenase, adenylate kinase release by membrane alterations and caspase 3/7 inductions. Cry1Ab caused cell death from 100 ppm. For Cry1Ac, under such conditions, no effects were detected. The Roundup tested alone from 1 to 20 000ppm is necrotic and apoptotic from 50ppm, far below agricultural dilutions (50% lethal concentration 57.5ppm). The only measured significant combined effect was that Cry1Ab and Cry1Ac reduced caspases 3/7 activations induced by Roundup; this could delay the activation of apoptosis. There was the same tendency for the other markers. In these results, we argue that modified Bt toxins are not inert on nontarget human cells, and that they can present combined sideeffects with other residues of pesticides specific to GM plants. Copyright © 2011 John Wiley & Sons, Ltd.
Keywords: Roundup; Bt toxins; Cry1Ab; Cry1Ac; GMOs; mixtures; glyphosate; human cells
INTRODUCTIONThe real effects of mixtures of chemical pollutants are a major concern for public health (Monosson, 2005). Humans are exposed to hundreds of compounds on a daily basis. The commercialized combinations could be a first matter of concern. Agricultural genetically modified organisms (GMOs) are steadily increasing worldwide, and they need to be carefully assessed (Séralini et al., 2009, 2011; Spiroux de Vendômois et al., 2010). Nowadays 99.9% of GMOs can be described as pesticide plants, designed for herbicide tolerance and/or modified insecticide production (James, 2010). Thus pesticides residues co-occur in the plant, synthesized by the plant itself, by the expression of the inserted transgene (modified Bt from Bacillus thuringiensis) or through external pesticide treatment facilitated by the transgene- dependent tolerance to herbicides (Roundup in most instances). In turn, such residues exert their effects upon consumption or release into the environment (Arregui et al, 2004; Tank et al., 2010). Owing to their key role in intensive agriculture, potential side effects of such combined pesticides residues should be assessed. In vitro tests are frequently recommended as a first step to replace animal models in toxicity studies. Here, we have tested for the first time the effects of Cry1Ab and Cry1Ac alone and combined with Roundup on human cells.
Modified toxins from Bt are Cry proteins forming pores in insect cell membranes (Then, 2010); they account for 39% of edible plant GMOs worldwide (James, 2010).
Since natural Bt toxins have long been used, their modified counterparts are often compared with them. However, the latter derivatives are truncated, adapted and modified synthetic sequences; consequently their activity is possibly quite different from the natural ones (Séralini et al., 2011). Also, Bt toxins are claimed and believed to be safe. Yet prions, hormones and venoms are also proteins, and are far from being innocuous. To date, Bt toxins have not been tested on human cells. However, Bt corns are regularly consumed by humans in America and their residues have even been found in maternal and fetal cord serum at around 0.2ppb (Aris and Leblanc, 2011), which does not take into account the tissue levels. Nontarget toxicity of natural Bt toxins has been detected in mammals, for instance at a 50% lethal concentration (LC50) from around 10 to 520ppb (Ito et al., 2004; Nagamatsu et al., 2010; Rani and Balaraman, 1996).Roundup formulations are mixtures of glyphosate and adjuvants such as ammonium sulfate, benzisothiazolone, glycerine, isobutane, isopropylamine, polyethoxylated alkylamines and sorbic acid (Cox, 2004). Glyphosate-based herbicides are the object of an increasing number of studies, which reveal, in combination with adjuvants, endocrine-disrupting effects, and tumor-promoting or teratogenic effects on numerous nontarget species (Gasnier et al., 2009; George et al., 2010; Paganelli et al., 2010), for instance from 0.5 ppm on androgen receptors. We have used human embryonic kidney cell line HEK293 as a sensitive model (Benachour and Séralini, 2009). The kidney model was used also because a body of evidence suggests kidney dysfunctions as endpoints of GMO diet effects (Séralini et al., 2011), and thus kidney cells could be a target for GMOs. We first measured the mitochondrial respiration level, by succinate dehydrogenase (SD) activity assessment in order to test cytotoxicity. Then, as Bt proteins act as pore forming toxins (Then, 2010), we determined adenylate kinase (AK) activity when released in the medium, revealing possible membrane alterations. In association, we assayed caspase 3 and 7 activities in order to separate the apoptotic and necrotic actions involved in cytotoxic effects. Moreover, human cell lines allow the study of unintended side effects on nontarget species of GMO-associated pesticides.
MATERIALS AND METHODS
ChemicalsCry1Ab and Cry1Ac were prepared as described previously by two different laboratories (Székács et al, 2010; Pusztai-Carey et al., 1994). The glyphosate-based herbicide tested was commercially available RoundupW GT Plus formulation, approval no. 2020448 (Monsanto, Anvers, Belgium). It contains 450 g l 1 glyphosate acid (N-phosphonomethyl-glycine). Successive dilutions were prepared in Eagle’s modified minimum essential medium (EMEM; Abcys, Paris, France). 3-(4,5-Dimethyl-thiazol-2-yl)- 2,5-diphenyl-tetrazolium bromide (MTT) and all other compounds were obtained from Sigma-Aldrich (Saint-Quentin Fallavier, France), unless specified. MTT was prepared as a 5mgml 1 stock solution in phosphate-buffered saline, filtered through a 0.22 mm filter before use, and diluted to 1mgml 1 in EMEM.
Toxin PreparationsThe Cry1Ab and Cry1Ac toxins are cloned from the natural Bacillus thuringiensis subspecies kurstaki HD-1 strain and expressed in Escherichia coli as single gene products. The inclusion bodies, containing the protoxins, were solubilized at pH 10.5 in the presence of b-mercaptoethanol and treated with commercial bovine trypsin (Sigma, USA). The 65 kDa activated toxins were isolated by ion exchange HPLC and the pure toxin fractions were desalted and lyophilized and stored at 80 C. After storage, toxins were diluted in a 50mM Na-carbonate–HCl buffer at 1mgml 1 (pH 9.5), and then diluted in EMEM.
Cell LinesThe human embryonic kidney 293 cell line (ECACC 85120602) was provided by Sigma-Aldrich (Saint-Quentin Fallavier, France). Cells were grown in phenol red-free EMEM (Abcys, Paris, France) containing 2mM glutamine, 1% nonessential amino acid, 100 U ml 1 of antibiotics (a mixture of penicillin, streptomycin and fungizone; Lonza, Saint Beauzire, France), 10mgml 1 of liquid kanamycin (Dominique Dutscher, Brumath, France) and 10% fetal bovine serum (PAA, les Mureaux, France). Cells were grown at 37 C (5% CO2, 95% air) during 24 h to 80% confluence, washed with serum-free EMEM and then exposed to various chemicals, since the serum delayed the cell necrosis by about 48 h in the presence of toxic compounds (Benachour et al., 2007). The control cells grow normally in serum-free medium up to 96 h.
Cell Treatments and Cytotoxicity BiomarkersCells at 80% confluence in 48- or 96-well plates (Dominique Dutscher, Brumath, France) were washed with serum-free EMEM, in order to avoid other combined effects, and then exposed to various concentrations of Bt toxins or Roundup GT Plus in EMEM serum-free medium for 24 h. Bt toxins were used from 10 ppb to 100 ppm (in the range of GM plant production). Concerning Roundup, 50% lethal concentrations (LC50) were assessed from 1 to 20 000 ppm (the latter is the agricultural dilution). Combined effects were measured by mixing LC50 of Roundup with three doses of each Bt toxin. After treatments, the following tests were applied: mitochondrial respiration assay (MTT) through the succinate dehydrogenase activity measurement (Mosmann, 1983). The optical density was measured at 570 nm using a Mithras LB 940 luminometer (Berthold, Thoiry, France). The bioluminescent ToxiLight bioassay (Lonza, Saint Beauzire, France) was applied for the membrane degradation assessment, by the intracellular AK release in the medium; this is described as a necrosis marker (Crouch et al., 1993). Finally, the apoptotic cell death was evaluated with the Caspase-Glo 3/7 assay (Promega, Paris, France). Luminescencewas measured using a Mithras LB 940 luminometer (Berthold, Thoiry, France). These methods were previously described by our group (Benachour and Séralini, 2009).
Statistical AnalysisThe experiments were repeated at least three times in different weeks on three independent cultures (n = 9). LC50 values were calculated by a nonlinear regression using a sigmoid (fiveparameter) equation with the GraphPad Prism 5 software. All data were presented as the means standard errors (SEs). Statistical differences were determined by Student t-test using significance levels at P<0.01 (**) and P<0.05 (*).
RESULTSWe measured for the first time cytotoxic effects of Bt toxins, alone or in combination with a glyphosate-based herbicide, on HEK293 cells. First of all, we confirmed that the buffer was not cytototoxic for the cells. The mitochondrial succinate dehydrogenase activity of treated cells significantly decreased at 100 ppm of Cry1Ab alone (by 11%, Fig. 1A). Even if it was limited, F1 this phenomenon was undetected for Cry1Ac. Lower doses were tested from 10 ppb to 10 ppm, but significant effects were not observed. We obtained similar effects with a Cry1Ab toxin provided by another laboratory that was prepared and stored independently (Fig. 1A). We measured AK activity after its release in the medium in order to evaluate plasma membrane integrity. A concentration of Cry1Ab of 100 ppm increased AK leakage in the medium 2-fold, revealing plasma membrane alterations. This was performed for the two Cry1Ab toxins. Apoptotic effects of Cry1Ab and Cry1Ac by means of caspase 3/7 activities were studied; no effects on HEK293 cells were visible. We can therefore confirm that Cry1Ab can induce cytotoxic effects via a necrotic mechanism in these conditions at 100 ppm.
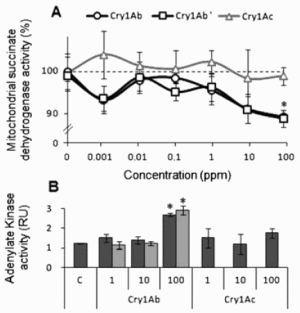 Figure 1. Cytotoxic effects of modified Bt toxins Cry1Ac and Cry1Ab on HEK293 cell line. (A) Cell death has been measured on mitochondrial succinate dehydrogenase after 24 h exposure to two Cry1Ab toxins up to 100 ppm prepared from different sources (A, black curves with circles and squares) and Cry1Ac (grey curve). (B) Cell membrane degradation was measured by adenylate kinase release (RU, relative units) provoked by Cry1Ab and Cry1Ac toxins (1–100 ppm) in comparison to control (C). No effect was detected on caspase 3/7 activities; therefore results are not displayed. Standard errors of the mean are indicated in all instances (n=9; * P<0.05).
Figure 1. Cytotoxic effects of modified Bt toxins Cry1Ac and Cry1Ab on HEK293 cell line. (A) Cell death has been measured on mitochondrial succinate dehydrogenase after 24 h exposure to two Cry1Ab toxins up to 100 ppm prepared from different sources (A, black curves with circles and squares) and Cry1Ac (grey curve). (B) Cell membrane degradation was measured by adenylate kinase release (RU, relative units) provoked by Cry1Ab and Cry1Ac toxins (1–100 ppm) in comparison to control (C). No effect was detected on caspase 3/7 activities; therefore results are not displayed. Standard errors of the mean are indicated in all instances (n=9; * P<0.05).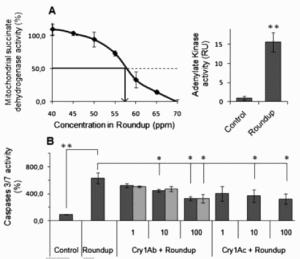 Figure 2. Effects of Roundup alone and with modified Bt toxins Cry1Ab and Cry1Ac on HEK293 cell line. (A) Cells were treated with Roundup (1–20 000 ppm) and the mitochondrial respiration was measured through succinate dehydrogenase activity (left). The LC50 for Roundup was determined as 57.5ppm by nonlinear regression (arrow). On the right, the adenylate kinase release showing membrane alterations by Roundup alone at its LC50 in comparison to control. (B) Combined effects of Roundup at its LC50 with Bt toxins measured on caspase 3/7 activities (%). The same tendency for the combined effects but with no significant results was detected on adenylate kinase release and succinate dehydrogenase; results not shown. Standards errors of the mean are indicated in all instances (n = 9). Significance of the effects (* P<0.05; ** P<0.01) is tested against the negative control C (medium alone) or the positive Roundup control (R).
Figure 2. Effects of Roundup alone and with modified Bt toxins Cry1Ab and Cry1Ac on HEK293 cell line. (A) Cells were treated with Roundup (1–20 000 ppm) and the mitochondrial respiration was measured through succinate dehydrogenase activity (left). The LC50 for Roundup was determined as 57.5ppm by nonlinear regression (arrow). On the right, the adenylate kinase release showing membrane alterations by Roundup alone at its LC50 in comparison to control. (B) Combined effects of Roundup at its LC50 with Bt toxins measured on caspase 3/7 activities (%). The same tendency for the combined effects but with no significant results was detected on adenylate kinase release and succinate dehydrogenase; results not shown. Standards errors of the mean are indicated in all instances (n = 9). Significance of the effects (* P<0.05; ** P<0.01) is tested against the negative control C (medium alone) or the positive Roundup control (R).Closer to the reality of exposure, we then tested combined F2 effects of Bt toxins with Roundup (Fig 2). According to our previous results, Roundup is cytotoxic by inhibition of mitochondrial respiration activity, far below agricultural dilutions (around 200 times less) with an LC50 of 57.5 ppm (Fig. 2A). At this concentration, Roundup also induced necrosis evidenced by a 15-fold increase of an AK release. Apoptosis induction was measured by a 6.7-fold caspase 3/7 activities enhancement (Fig. 2B). However, concerning combined effects we observed significant effects on apoptosis; both Bt toxins from 10 ppm reduced caspase 3/7 activities (by around 50%) when they were induced in Roundup at its LC50 (Fig. 2B). Similarly, there was a non significant tendency for both toxins (data not shown) to reduce AK leakage and mitochondrial respiration inhibition induced by Roundup.
DISCUSSIONFew studies have been performed on nontarget effects of Bt toxins, and none with modified Bt toxins extracted from plants, or together with Roundup residues, even in regulatory files. For natural Bt toxins, their mechanisms of action and insect resistance are not fully understood (Singh and Sivaprasad, 2009), and the metabolism of these proteins in mammals is unknown (Séralini et al., 2011; Chowdhury et al., 2003). They may even interact with extrinsic factors (Then, 2010). Billions of people and wildlife could be exposed to modified Bt toxins; therefore understanding their potential side effects is crucial.
On two biomarkers of cell death, Cry1Ab exposure led to respiration inhibition and plasma membrane alterations, by contrast to Cry1Ac. This could be consistent with the fact that the consumption of MON810 maize producing Cry1Ab (in the ppm range) induced signs of hepatorenal alterations in a subchronic feeding study on rats (Spiroux de Vendômois et al., 2009). It is known that both toxins differ significantly in their domain III structure (Karim and Dean, 2000), which is the only one to be involved at the same time in ion channel function, receptor binding and insertion into the membrane (Dean et al., 1996). This occurred at relatively high concentrations (100 ppm) in comparison to the concentrations produced in GM plants (1–20 ppm, Székács et al., 2010). The content can differ greatly according to the GM variety and environmental conditions (Then and Lorch, 2008). The exposure during consumption can appear low enough to avoid side effects, and whether this occurs in vivo remains to be checked. However, the bioaccumulation in tissues, or bioaccumulative or long-term effects, has to be taken into account since Bt residues were recently claimed to be measured in pregnant women’s serum at around 0.2 ppb (Aris and Leblanc, 2011). In addition, high quantities of Bt crops can be consumed by mammals. The procedure for GMO market authorizations for crops such as MON810 (EFSA, 2009) does not require in vitro tests on human cells of Bt toxins, nor on its combinatorial effects, thus our results are raising new questions about the safety of these toxins and the Bt crops in general. Although in vitro studies suggest degradation in human gastric secretions, digestion is never a complete process and insecticide Q2 toxins cannot be fully degraded in vivo (Paul et al., 2010). This is known to be the case for Cry1Ab (Chowdhury et al., 2003). It must be underlined that the insecticidal proteins produced by the GM plants are in soluble forms, and thus already biochemically activated, unlike those produced by the microorganism Bt, secreted as inactive precursors or protoxins (Hilbeck and Schmidt, 2006). The importance of Bt toxin activation has been demonstrated in relation to in vitro membrane damages of human erythrocytes, by solubilized Bt toxins, but not by the intact form (Rani and Balaraman, 1996). Cellular response to Bt toxins does not elicit apoptosis; it induces necrotic effects via a plasma membrane disruption for Cry1Ab within only 24 h. This may be due to pore formation like in insect cells owing to binding to specific receptors or membrane lipid rafts (Then, 2010; Soberón et al., 2009).
We also demonstrated that Cry1Ab and Cry1Ac exposures slightly reduced caspase 3/7 activations induced by Roundup. This could be related at least in part to the properties of Roundup compounds, especially adjuvants. We observed previously, in our group, that serum delayed the cytotoxic effects induced by Roundup. This was probably due to serum binding proteins (Benachour et al., 2007). Here we can assume that physico- chemical properties of proteins may give them the ability to bind and form complexes with Roundup adjuvants that have tendencies to form vesicles, buffering their bioavailability to cells. Similarly, a nonsignificant tendency of reduction of the cytotoxic effects of Roundup was observed on mitochondrial respiration and membrane degradation when the toxins were added. The apoptosis induction appeared to be the most sensitive impact of combined effects. This does not exclude other intracellular targets such as endocrine disruption, since Roundup is antiandrogenic from 0.5 ppm, below toxic levels and close to human serum levels (0.1–0.2 ppm in Acquavella et al., 2004).
Here we documented that modified Bt toxins are not inert on human cells, but can exert toxicity, at least under certain in vitro conditions. In vivo implications should be now assessed. Our results raise new questions in the risk assessment of food and feed derived from genetically engineered plants.
AcknowledgmentsWe are grateful to Testbiotech and the GEKKO Foundation, the association Denis Guichard, CRIIGEN and the Conseil Regional of Basse-Normandie for their support. R.M. and E.C. are recipients of fellowships from CRIIGEN and the Conseil Regional of Basse- Normandie. We would also like to thank Marianne Pusztai-Carey for one of the toxin preparations.
REFERENCESAcquavella JF, Alexander BH, Mandel JS, Gustin C, Baker B, Chapman P, Bleeke M. 2004. Glyphosate biomonitoring for farmers and their families: results from the Farm Family Exposure Study. Environ. Health Perspect. 112(3): 321–326.
Q3 Aris A, Leblanc S. 2011. Maternal and fetal exposure to pesticides associated to genetically modified foods in Eastern Townships of Quebec, Canada. Reprod. Toxicol., February 18.
Arregui MC, Lenardon A, Sanchez D, Maitre MI, Scotta R, Enrique S. 2004. Monitoring glyphosate residues in transgenic glyphosate-resistant soybean. Pest Manag. Sci. 60: 163–166.
Benachour N, Séralini GE. 2009. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem. Res. Toxicol. 22: 97–105.
Benachour N, Sipahutar H, Moslemi S, Gasnier C, Travert C, Séralini GE. 2007. Time- and dose-dependent effects of roundup on human embryonic and placental cells. Arch. Environ. Contam. Toxicol. 53: 126–133.
Chowdhury EH, Shimada N, Murata H, Mikami O, Sultana P, Miyazaki S, Yoshioka M, Yamanaka N, Hirai N, Nakajima Y. 2003. Detection of Cry1Ab protein in gastrointestinal contents but not visceral organs of genetically modified Bt11-fed calves. Vet. Hum. Toxicol. 45: 72–75.
Cox C. 2004. Glyphosate. J. Pest. Reform. 24: 10–15.
Crouch SP, Kozlowski R, Slater KJ, Fletcher J. 1993. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Meth. 160: 81–88.
Dean DH, Rajamohan F, Lee MK, Wu SJ, Chen XJ, Alcantara E, Hussain SR. 1996. Probing the mechanism of action of Bacillus thuringiensis insecticidal proteins by site-directed mutagenesis – a minireview. Gene 179(1): 111–117.
EFSA. 2009. Scientific opinion on genetically modified organism MON810. The EFSA J. 1149: 1–84.
Gasnier C, Dumont C, Benachour N, Clair E, Chagnon MC, Seralini GE. 2009. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 262: 184–191.
George J, Prasad S, Mahmood Z, Shukla Y. 2010. Studies on glyphosateinduced carcinogenicity in mouse skin: a proteomic approach. J. Proteom. 73: 951–964.
Hilbeck A, Schmidt JEU. 2006. Another view on Bt proteins – how specific are they and what else might they do? Biopest. Int. 2(1): 1–50.
Ito A, Sasaguri Y, Kitada S, Kusaka Y, Kuwano K, Masutomi K, Mizuki E, Akao T, Ohba M. 2004. A Bacillus thuringiensis crystal protein with selective cytocidal action to human cells. J. Biol. Chem. 279: 21282– 21286.
James C. 2010. Global status of commercialized biotech/GM crops: 2010 ISAAA Brief 42.
Karim S, Dean DH. 2000. Pesticidal and receptor binding properties of Bacillus thuringiensis Cry1Ab and Cry1Ac delta-endotoxin mutants to Pectinophora gossypiella and Helicoverpa zea. Curr. Microbiol. 41(6): 430–440.
Monosson E. 2005. Chemical mixtures: considering the evolution of toxicology and chemical assessment. Environ. Health. Perspect. 113: 383–390.
Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 65: 55–63.
Nagamatsu Y, Okamura S, Saitou H, Akao T, Mizuki E. 2010. Three Cry toxins in two types from Bacillus thuringiensis strain M019 preferentially kill human hepatocyte cancer and uterus cervix cancer cells. Biosci. Biotechnol. Biochem. 74: 494–498.
Paganelli A, Gnazzo V, Acosta H, Lopez SL, Carrasco AE. 2010. Glyphosatebased herbicides produce teratogenic effects on vertebrates by impairing retinoic acid signaling. Chem. Res. Toxicol. 23: 1586–1595.
Paul V, Guertler P, Wiedemann S, Meyer HH. 2010. Degradation of Cry1Ab protein from genetically modified maize (MON810) in relation to total dietary feed proteins in dairy cow digestion. Transgenic Res. 19: 683–689.
Pusztai-Carey M, Carey P, Lessard T, Yaguchi M. 1994. Isolation, quantitation and purification of insecticidal proteins from Bacillus thuringiensis. US patent 5,356,788.
Rani SS, Balaraman K. 1996. Effect of insecticidal crystal proteins of Bacillus thuringiensis on human erythrocytes in vitro. Indian J. Exp. Biol. 34: 1241–1244.
Séralini GE, Spiroux de Vendômois J, Cellier D, Sultan C, BuiattiM, Gallagher L, Antoniou M, Dronamraju KR. 2009. How subchronic and chronic health effects can be neglected for GMOs, pesticides or chemicals. Int. J. Biol. Sci. 5: 438–443.
Séralini GE, Mesnage R, Clair E, Gress S, Cellier D, Spiroux de Vendômois J. 2011. Genetically modified crops safety assessments: present limits and possible improvements. Environ. Sci. Eur. 23: 10.
Singh A, Sivaprasad CV. 2009. Functional interpretation of APN receptor from M. sexta using a molecular model. Bioinformation 3: 321–325.
Soberón M, Gill SS, Bravo A. 2009. Signaling versus punching hole: how do Bacillus thuringiensis toxins kill insect midgut cells. Cell Mol. Life Sci. 66: 1337–1349.
Spiroux de Vendômois J, Roullier F, Cellier D, Séralini GE. 2009. A comparison of the effects of three GM corn varieties on mammalian health. Int. J. Biol. Sci. 5: 706–726.
Spiroux de Vendômois J, Cellier D, Vélot C, Clair E, Mesnage R, Séralini GE. 2010. Debate on GMOs health risks after statistical findings in regulatory tests. Int. J. Biol. Sci. 6: 590–598.
Székács A, Lauber E, Juracsek J, Darvas B. 2010. Cry1Ab toxin production of MON 810 transgenic maize. Environ. Toxicol. Chem. 29: 182–190.
Tank JL, Rosi-Marshall EJ, Royer TV, Whiles MR, Griffiths NA, Frauendorf TC, Treering DJ. 2010. Occurrence of maize detritus and a transgenic insecticidal protein (Cry1Ab) within the stream network of an agricultural landscape. Proc. Natl Acad. Sci. USA 107: 17645–17650.
Then C. 2010. Risk assessment of toxins derived fromBacillus thuringiensis – synergism, efficacy, and selectivity. Environ. Sci. Pollut. Res. Int. 17: 791–797.
Then C, Lorch A. 2008. A simple question in a complex environment: How much Bt toxin do genetically engineered MON810 maize plants actually produce? Theor. Ökol. 14: 1–5.
_______________
Notes:*Correspondence to: G.-E. Séralini, University of Caen, Risk Pole MRSH-CNRS, Laboratory of Biochemistry EA2608, Esplanade de la Paix, 14032 Caen cedex, France. E-mail:
criigen@unicaen.fraUniversity of Caen, Risk Pole MRSH-CNRS, Laboratory of Biochemistry EA2608, Esplanade de la Paix, 14032 Caen cedex, France
bCRIIGEN, 40 rue de Monceau, 75008 Paris, France
cTestbiotech e.V., Frohschammerstr. 14, 80807 München, Germany
dPlant Protection Institute, Hungarian Academy of Sciences, H-1525, Budapest, POB 102, Hungary


