by Robert F. Kennedy Jr.
6/21/05
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT
YOU ARE REQUIRED TO READ THE COPYRIGHT NOTICE AT THIS LINK BEFORE YOU READ THE FOLLOWING WORK, THAT IS AVAILABLE SOLELY FOR PRIVATE STUDY, SCHOLARSHIP OR RESEARCH PURSUANT TO 17 U.S.C. SECTION 107 AND 108. IN THE EVENT THAT THE LIBRARY DETERMINES THAT UNLAWFUL COPYING OF THIS WORK HAS OCCURRED, THE LIBRARY HAS THE RIGHT TO BLOCK THE I.P. ADDRESS AT WHICH THE UNLAWFUL COPYING APPEARED TO HAVE OCCURRED. THANK YOU FOR RESPECTING THE RIGHTS OF COPYRIGHT OWNERS.
When a study revealed that mercury in childhood vaccines may have caused autism in thousands of kids, the government rushed to conceal the data -- and to prevent parents from suing drug companies for their role in the epidemic.
In June 2000, a group of top government scientists and health officials gathered for a meeting at the isolated Simpsonwood conference center in Norcross, Ga. Convened by the Centers for Disease Control and Prevention, the meeting was held at this Methodist retreat center, nestled in wooded farmland next to the Chattahoochee River, to ensure complete secrecy. The agency had issued no public announcement of the session -- only private invitations to 52 attendees. There were high-level officials from the CDC and the Food and Drug Administration, the top vaccine specialist from the World Health Organization in Geneva, and representatives of every major vaccine manufacturer, including GlaxoSmithKline, Merck, Wyeth and Aventis Pasteur. All of the scientific data under discussion, CDC officials repeatedly reminded the participants, was strictly "embargoed." There would be no making photocopies of documents, no taking papers with them when they left.
The federal officials and industry representatives had assembled to discuss a disturbing new study that raised alarming questions about the safety of a host of common childhood vaccines administered to infants and young children. According to a CDC epidemiologist named Tom Verstraeten, who had analyzed the agency's massive database containing the medical records of 100,000 children, a mercury-based preservative in the vaccines -- thimerosal -- appeared to be responsible for a dramatic increase in autism and a host of other neurological disorders among children. "I was actually stunned by what I saw," Verstraeten told those assembled at Simpsonwood, citing the staggering number of earlier studies that indicate a link between thimerosal and speech delays, attention-deficit disorder, hyperactivity and autism. Since 1991, when the CDC and the FDA had recommended that three additional vaccines laced with the preservative be given to extremely young infants -- in one case, within hours of birth -- the estimated number of cases of autism had increased fifteenfold, from one in every 2,500 children to one in 166 children.
Even for scientists and doctors accustomed to confronting issues of life and death, the findings were frightening. "You can play with this all you want," Dr. Bill Weil, a consultant for the American Academy of Pediatrics, told the group. The results "are statistically significant." Dr. Richard Johnston, an immunologist and pediatrician from the University of Colorado whose grandson had been born early on the morning of the meeting's first day, was even more alarmed. "My gut feeling?" he said. "Forgive this personal comment -- I do not want my grandson to get a thimerosal-containing vaccine until we know better what is going on."
But instead of taking immediate steps to alert the public and rid the vaccine supply of thimerosal, the officials and executives at Simpsonwood spent most of the next two days discussing how to cover up the damaging data. According to transcripts obtained under the Freedom of Information Act, many at the meeting were concerned about how the damaging revelations about thimerosal would affect the vaccine industry's bottom line.
"We are in a bad position from the standpoint of defending any lawsuits," said Dr. Robert Brent, a pediatrician at the Alfred I. duPont Hospital for Children in Delaware. "This will be a resource to our very busy plaintiff attorneys in this country." Dr. Bob Chen, head of vaccine safety for the CDC, expressed relief that "given the sensitivity of the information, we have been able to keep it out of the hands of, let's say, less responsible hands." Dr. John Clements, vaccines advisor at the World Health Organization, declared flatly that the study "should not have been done at all" and warned that the results "will be taken by others and will be used in ways beyond the control of this group. The research results have to be handled."
In fact, the government has proved to be far more adept at handling the damage than at protecting children's health. The CDC paid the Institute of Medicine to conduct a new study to whitewash the risks of thimerosal, ordering researchers to "rule out" the chemical's link to autism. It withheld Verstraeten's findings, even though they had been slated for immediate publication, and told other scientists that his original data had been "lost" and could not be replicated. And to thwart the Freedom of Information Act, it handed its giant database of vaccine records over to a private company, declaring it off-limits to researchers. By the time Verstraeten finally published his study in 2003, he had gone to work for GlaxoSmithKline and reworked his data to bury the link between thimerosal and autism.
Vaccine manufacturers had already begun to phase thimerosal out of injections given to American infants -- but they continued to sell off their mercury-based supplies of vaccines until last year. The CDC and FDA gave them a hand, buying up the tainted vaccines for export to developing countries and allowing drug companies to continue using the preservative in some American vaccines -- including several pediatric flu shots as well as tetanus boosters routinely given to 11-year-olds.
The drug companies are also getting help from powerful lawmakers in Washington. Senate Majority Leader Bill Frist, who has received $873,000 in contributions from the pharmaceutical industry, has been working to immunize vaccine makers from liability in 4,200 lawsuits that have been filed by the parents of injured children. On five separate occasions, Frist has tried to seal all of the government's vaccine-related documents -- including the Simpsonwood transcripts -- and shield Eli Lilly, the developer of thimerosal, from subpoenas. In 2002, the day after Frist quietly slipped a rider known as the "Eli Lilly Protection Act" into a homeland security bill, the company contributed $10,000 to his campaign and bought 5,000 copies of his book on bioterrorism. Congress repealed the measure in 2003 -- but earlier this year, Frist slipped another provision into an anti-terrorism bill that would deny compensation to children suffering from vaccine-related brain disorders. "The lawsuits are of such magnitude that they could put vaccine producers out of business and limit our capacity to deal with a biological attack by terrorists," says Andy Olsen, a legislative assistant to Frist.
Even many conservatives are shocked by the government's effort to cover up the dangers of thimerosal. Rep. Dan Burton, a Republican from Indiana, oversaw a three-year investigation of thimerosal after his grandson was diagnosed with autism. "Thimerosal used as a preservative in vaccines is directly related to the autism epidemic," his House Government Reform Committee concluded in its final report. "This epidemic in all probability may have been prevented or curtailed had the FDA not been asleep at the switch regarding a lack of safety data regarding injected thimerosal, a known neurotoxin." The FDA and other public-health agencies failed to act, the committee added, out of "institutional malfeasance for self protection" and "misplaced protectionism of the pharmaceutical industry."
The story of how government health agencies colluded with Big Pharma to hide the risks of thimerosal from the public is a chilling case study of institutional arrogance, power and greed. I was drawn into the controversy only reluctantly. As an attorney and environmentalist who has spent years working on issues of mercury toxicity, I frequently met mothers of autistic children who were absolutely convinced that their kids had been injured by vaccines. Privately, I was skeptical. I doubted that autism could be blamed on a single source, and I certainly understood the government's need to reassure parents that vaccinations are safe; the eradication of deadly childhood diseases depends on it. I tended to agree with skeptics like Rep. Henry Waxman, a Democrat from California, who criticized his colleagues on the House Government Reform Committee for leaping to conclusions about autism and vaccinations. "Why should we scare people about immunization," Waxman pointed out at one hearing, "until we know the facts?"
It was only after reading the Simpsonwood transcripts, studying the leading scientific research and talking with many of the nation's preeminent authorities on mercury that I became convinced that the link between thimerosal and the epidemic of childhood neurological disorders is real. Five of my own children are members of the Thimerosal Generation -- those born between 1989 and 2003 -- who received heavy doses of mercury from vaccines. "The elementary grades are overwhelmed with children who have symptoms of neurological or immune-system damage," Patti White, a school nurse, told the House Government Reform Committee in 1999. "Vaccines are supposed to be making us healthier; however, in 25 years of nursing I have never seen so many damaged, sick kids. Something very, very wrong is happening to our children." More than 500,000 kids currently suffer from autism, and pediatricians diagnose more than 40,000 new cases every year. The disease was unknown until 1943, when it was identified and diagnosed among 11 children born in the months after thimerosal was first added to baby vaccines in 1931.
Some skeptics dispute that the rise in autism is caused by thimerosal-tainted vaccinations. They argue that the increase is a result of better diagnosis -- a theory that seems questionable at best, given that most of the new cases of autism are clustered within a single generation of children. "If the epidemic is truly an artifact of poor diagnosis," scoffs Dr. Boyd Haley, one of the world's authorities on mercury toxicity, "then where are all the 20-year-old autistics?" Other researchers point out that Americans are exposed to a greater cumulative "load" of mercury than ever before, from contaminated fish to dental fillings, and suggest that thimerosal in vaccines may be only part of a much larger problem. It's a concern that certainly deserves far more attention than it has received -- but it overlooks the fact that the mercury concentrations in vaccines dwarf other sources of exposure to our children.
What is most striking is the lengths to which many of the leading detectives have gone to ignore -- and cover up -- the evidence against thimerosal. From the very beginning, the scientific case against the mercury additive has been overwhelming. The preservative, which is used to stem fungi and bacterial growth in vaccines, contains ethylmercury, a potent neurotoxin. Truckloads of studies have shown that mercury tends to accumulate in the brains of primates and other animals after they are injected with vaccines -- and that the developing brains of infants are particularly susceptible. In 1977, a Russian study found that adults exposed to much lower concentrations of ethylmercury than those given to American children still suffered brain damage years later. Russia banned thimerosal from children's vaccines 20 years ago, and Denmark, Austria, Japan, Great Britain and all the Scandinavian countries have since followed suit.
"You couldn't even construct a study that shows thimerosal is safe," says Haley, who heads the chemistry department at the University of Kentucky. "It's just too darn toxic. If you inject thimerosal into an animal, its brain will sicken. If you apply it to living tissue, the cells die. If you put it in a petri dish, the culture dies. Knowing these things, it would be shocking if one could inject it into an infant without causing damage."
Internal documents reveal that Eli Lilly, which first developed thimerosal, knew from the start that its product could cause damage -- and even death -- in both animals and humans. In 1930, the company tested thimerosal by administering it to 22 patients with terminal meningitis, all of whom died within weeks of being injected -- a fact Lilly didn't bother to report in its study declaring thimerosal safe. In 1935, researchers at another vaccine manufacturer, Pittman-Moore, warned Lilly that its claims about thimerosal's safety "did not check with ours." Half the dogs Pittman injected with thimerosal-based vaccines became sick, leading researchers there to declare the preservative "unsatisfactory as a serum intended for use on dogs."
In the decades that followed, the evidence against thimerosal continued to mount. During the Second World War, when the Department of Defense used the preservative in vaccines on soldiers, it required Lilly to label it "poison." In 1967, a study in Applied Microbiology found that thimerosal killed mice when added to injected vaccines. Four years later, Lilly's own studies discerned that thimerosal was "toxic to tissue cells" in concentrations as low as one part per million -- 100 times weaker than the concentration in a typical vaccine. Even so, the company continued to promote thimerosal as "nontoxic" and also incorporated it into topical disinfectants. In 1977, 10 babies at a Toronto hospital died when an antiseptic preserved with thimerosal was dabbed onto their umbilical cords.
In 1982, the FDA proposed a ban on over-the-counter products that contained thimerosal, and in 1991 the agency considered banning it from animal vaccines. But tragically, that same year, the CDC recommended that infants be injected with a series of mercury-laced vaccines. Newborns would be vaccinated for hepatitis B within 24 hours of birth, and 2-month-old infants would be immunized for haemophilus influenzae B and diphtheria-tetanus-pertussis.
The drug industry knew the additional vaccines posed a danger. The same year that the CDC approved the new vaccines, Dr. Maurice Hilleman, one of the fathers of Merck's vaccine programs, warned the company that 6-month-olds who were administered the shots would suffer dangerous exposure to mercury. He recommended that thimerosal be discontinued, "especially when used on infants and children," noting that the industry knew of nontoxic alternatives. "The best way to go," he added, "is to switch to dispensing the actual vaccines without adding preservatives."
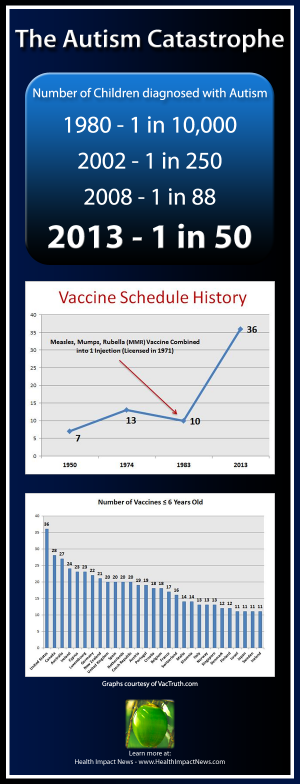
For Merck and other drug companies, however, the obstacle was money. Thimerosal enables the pharmaceutical industry to package vaccines in vials that contain multiple doses, which require additional protection because they are more easily contaminated by multiple needle entries. The larger vials cost half as much to produce as smaller, single-dose vials, making it cheaper for international agencies to distribute them to impoverished regions at risk of epidemics. Faced with this "cost consideration," Merck ignored Hilleman's warnings, and government officials continued to push more and more thimerosal-based vaccines for children. Before 1989, American preschoolers received only three vaccinations -- for polio, diphtheria-tetanus-pertussis and measlesmumps- rubella. A decade later, thanks to federal recommendations, children were receiving a total of 22 immunizations by the time they reached first grade.
As the number of vaccines increased, the rate of autism among children exploded. During the 1990s, 40 million children were injected with thimerosal-based vaccines, receiving unprecedented levels of mercury during a period critical for brain development. Despite the well-documented dangers of thimerosal, it appears that no one bothered to add up the cumulative dose of mercury that children would receive from the mandated vaccines. "What took the FDA so long to do the calculations?" Peter Patriarca, director of viral products for the agency, asked in an e-mail to the CDC in 1999. "Why didn't CDC and the advisory bodies do these calculations when they rapidly expanded the childhood immunization schedule?"
But by that time, the damage was done. Infants who received all their vaccines, plus boosters, by the age of 6 months were being injected with levels of ethylmercury 187 times greater than the EPA's limit for daily exposure to methylmercury, a related neurotoxin. Although the vaccine industry insists that ethylmercury poses little danger because it breaks down rapidly and is removed by the body, several studies -- including one published in April by the National Institutes of Health -- suggest that ethylmercury is actually more toxic to developing brains and stays in the brain longer than methylmercury.
Officials responsible for childhood immunizations insist that the additional vaccines were necessary to protect infants from disease and that thimerosal is still essential in developing nations, which, they often claim, cannot afford the single-dose vials that don't require a preservative. Dr. Paul Offit, one of CDC's top vaccine advisors, told me, "I think if we really have an influenza pandemic -- and certainly we will in the next 20 years, because we always do -- there's no way on God's earth that we immunize 280 million people with single-dose vials. There has to be multidose vials."
For years some parents and scientists have raised concerns about vaccine safety, including a possible link to autism and ADD. Many independent experts have sided with government officials and other scientists who say there's no possible connection. But how "independent" are they? CBS News investigative correspondent Sharyl Attkisson shares here what she found.
They're some of the most trusted voices in the defense of vaccine safety: the American Academy of Pediatrics, Every Child By Two, and pediatrician Dr. Paul Offit.
But CBS News has found these three have something more in common -- strong financial ties to the industry whose products they promote and defend.
The vaccine industry gives millions to the Academy of Pediatrics for conferences, grants, medical education classes and even helped build their headquarters. The totals are kept secret, but public documents reveal bits and pieces.
• A $342,000 payment from Wyeth, maker of the pneumococcal vaccine -- which makes $2 billion a year in sales.
• A $433,000 contribution from Merck, the same year the academy endorsed Merck's HPV vaccine -- which made $1.5 billion a year in sales.
• Another top donor: Sanofi Aventis, maker of 17 vaccines and a new five-in-one combo shot just added to the childhood vaccine schedule last month.
Every Child By Two, a group that promotes early immunization for all children, admits the group takes money from the vaccine industry, too -- but wouldn't tell us how much.
A spokesman told CBS News: "There are simply no conflicts to be unearthed." But guess who's listed as the group's treasurers? Officials from Wyeth and a paid advisor to big pharmaceutical clients.
Then there's Paul Offit, perhaps the most widely-quoted defender of vaccine safety.
He's gone so far as to say babies can tolerate "10,000 vaccines at once."
This is how Offit described himself in a previous interview: "I'm the chief of infectious disease at Children's Hospital of Philadelphia and a professor of pediatrics at Penn's medical school," he said.
Offit was not willing to be interviewed on this subject but like others in this CBS News investigation, he has strong industry ties. In fact, he's a vaccine industry insider.
Offit holds in a $1.5 million dollar research chair at Children's Hospital, funded by Merck. He holds the patent on an anti-diarrhea vaccine he developed with Merck, Rotateq, which has prevented thousands of hospitalizations.On March 22, 2010, Food and Drug Administration (FDA) officials, adhering to the precautionary principle, advised American doctors to suspend use of Rotarix 1 vaccine until the agency finds out why DNA from a swine virus (porcine circovirus 1 or PCV1) was found in the live rotavirus vaccine. The FDA said there is “no evidence at this time” that the vaccine manufactured by GlaxoSmithKline and given to babies at 2,4 and 6 months of age to prevent diarrhea poses any safety risk. 2
Independent Lab Using New Technology Found Contamination
The discovery that viral DNA is contaminating Rotarix vaccine was made by a team of scientists at an independent research lab in San Fransisco, California, where they used new technology to detect fragments of viral genetic material in vaccines using genetic sequencing. 3
More testing confirmed that many copies of DNA from the pig virus were present in all Rotarix vaccine lots released since the vaccine was licensed in 2008 because the pig virus DNA also contaminated the working cell bank and the original viral “seed” stock, from which Rotarix vaccine was first produced. 4
Two Other Live Virus Vaccines Contaminated
The surprising discovery reportedly was made after the independent lab used new technology to evaluate the purity of eight live virus vaccines for polio, rubella, measles, yellow fever, human herpes 3 (varicella or chicken pox), rotavirus (Rotarix and RotaTeq) and MMR. In addition to pig viral DNA found in Rotarix vaccine, low levels of DNA fragments from avian (bird) leukosis virus (a retrovirus) was found in measles vaccine and DNA fragments of a virus similar to simian (monkey) retrovirus was found in RotaTeq vaccine. 5
-- Vaccine Contamination: Pig Virus DNA Found in Rotarix, by Barbara Loe Fisher
And future royalties for the vaccine were just sold for $182 million cash. Dr. Offit's share of vaccine profits? Unknown.
There's nothing illegal about the financial relationships, but to critics, they pose a serious risk for conflicts of interest. As one member of Congress put it, money from the pharmaceutical industry can shape the practices of those who hold themselves out to be "independent."
The American Academy of Pediatrics, Every Child By Two and Dr. Offit would not agree to interviews, but all told us they're up front about the money they receive, and it doesn't sway their opinions.
-- How Independent Are Vaccine Defenders?, by Sharyl Attkisson
But while public-health officials may have been well-intentioned, many of those on the CDC advisory committee who backed the additional vaccines had close ties to the industry. Dr. Sam Katz, the committee's chair, was a paid consultant for most of the major vaccine makers and shares a patent on a measles vaccine with Merck, which also manufactures the hepatitis B vaccine. Dr. Neal Halsey, another committee member, worked as a researcher for the vaccine companies and received honoraria from Abbott Labs for his research on the hepatitis B vaccine.
Q. Walter, what does that tell you? Someone’s fanny ought to be sitting in a jail cell, I say. What do you suggest?
A. That the Chairman of the American of the American Academy of Pediatrics, Dr. Sam Katz, should be in jail with them. I say this based on the internal memorandums at Lederle, which indicate they obtained his support to block regulations that would have required Lederle to remove the simian herpes viruses from their vaccine after it was discovered in 1971 that 100% of the OPV carried African green monkey cytomegalovirus within the vaccine. I say this without regard to his giving me one of the highest accolades a vaccine injury attorney could receive when he shouted at me (after Congressman Burton’s hearing on the Hep B vaccine) – “You are a despicable attorney.”
Katz co-authored the FDA treatise on polio vaccines in 1980 that asserted the regulations did not prohibit release of oral polio vaccines containing simian retroviruses, which included Simian immunodeficiency viruses from rhesus monkeys, African green monkeys and chimpanzees that could be passed by the vaccine.
-- Exposing The FDA’s Vaccine Injury Cover-Up: An Interview With Walter Kyle, Esq., by Catherine J. Frompovich
Indeed, in the tight circle of scientists who work on vaccines, such conflicts of interest are common. Rep. Burton says that the CDC "routinely allows scientists with blatant conflicts of interest to serve on intellectual advisory committees that make recommendations on new vaccines," even though they have "interests in the products and companies for which they are supposed to be providing unbiased oversight." The House Government Reform Committee discovered that four of the eight CDC advisors who approved guidelines for a rotavirus vaccine laced with thimerosal "had financial ties to the pharmaceutical companies that were developing different versions of the vaccine."
Offit, who shares a patent on the vaccine, acknowledged to me that he "would make money" if his vote to approve it eventually leads to a marketable product. But he dismissed my suggestion that a scientist's direct financial stake in CDC approval might bias his judgment. "It provides no conflict for me," he insists. "I have simply been informed by the process, not corrupted by it. When I sat around that table, my sole intent was trying to make recommendations that best benefited the children in this country. It's offensive to say that physicians and public-health people are in the pocket of industry and thus are making decisions that they know are unsafe for children. It's just not the way it works."
Other vaccine scientists and regulators gave me similar assurances. Like Offit, they view themselves as enlightened guardians of children's health, proud of their "partnerships" with pharmaceutical companies, immune to the seductions of personal profit, besieged by irrational activists whose anti-vaccine campaigns are endangering children's health. They are often resentful of questioning. "Science," says Offit, "is best left to scientists."
Still, some government officials were alarmed by the apparent conflicts of interest. In his e-mail to CDC administrators in 1999, Paul Patriarca of the FDA blasted federal regulators for failing to adequately scrutinize the danger posed by the added baby vaccines. "I'm not sure there will be an easy way out of the potential perception that the FDA, CDC and immunization-policy bodies may have been asleep at the switch re: thimerosal until now," Patriarca wrote. The close ties between regulatory officials and the pharmaceutical industry, he added, "will also raise questions about various advisory bodies regarding aggressive recommendations for use" of thimerosal in child vaccines.
If federal regulators and government scientists failed to grasp the potential risks of thimerosal over the years, no one could claim ignorance after the secret meeting at Simpsonwood. But rather than conduct more studies to test the link to autism and other forms of brain damage, the CDC placed politics over science. The agency turned its database on childhood vaccines -- which had been developed largely at taxpayer expense -- over to a private agency, America's Health Insurance Plans, ensuring that it could not be used for additional research. It also instructed the Institute of Medicine, an advisory organization that is part of the National Academy of Sciences, to produce a study debunking the link between thimerosal and brain disorders. The CDC "wants us to declare, well, that these things are pretty safe," Dr. Marie McCormick, who chaired the IOM's Immunization Safety Review Committee, told her fellow researchers when they first met in January 2001. "We are not ever going to come down that [autism] is a true side effect" of thimerosal exposure. According to transcripts of the meeting, the committee's chief staffer, Kathleen Stratton, predicted that the IOM would conclude that the evidence was "inadequate to accept or reject a causal relation" between thimerosal and autism. That, she added, was the result "Walt wants" -- a reference to Dr. Walter Orenstein, director of the National Immunization Program for the CDC.
For those who had devoted their lives to promoting vaccination, the revelations about thimerosal threatened to undermine everything they had worked for. "We've got a dragon by the tail here," said Dr. Michael Kaback, another committee member. "The more negative that [our] presentation is, the less likely people are to use vaccination, immunization -- and we know what the results of that will be. We are kind of caught in a trap. How we work our way out of the trap, I think is the charge."
Even in public, federal officials made it clear that their primary goal in studying thimerosal was to dispel doubts about vaccines. "Four current studies are taking place to rule out the proposed link between autism and thimerosal," Dr. Gordon Douglas, then-director of strategic planning for vaccine research at the National Institutes of Health, assured a Princeton University gathering in May 2001. "In order to undo the harmful effects of research claiming to link the [measles] vaccine to an elevated risk of autism, we need to conduct and publicize additional studies to assure parents of safety." Douglas formerly served as president of vaccinations for Merck, where he ignored warnings about thimerosal's risks.
In May of last year, the Institute of Medicine issued its final report. Its conclusion: There is no proven link between autism and thimerosal in vaccines. Rather than reviewing the large body of literature describing the toxicity of thimerosal, the report relied on four disastrously flawed epidemiological studies examining European countries, where children received much smaller doses of thimerosal than American kids. It also cited a new version of the Verstraeten study, published in the journal Pediatrics, that had been reworked to reduce the link between thimerosal and autism. The new study included children too young to have been diagnosed with autism and overlooked others who showed signs of the disease. The IOM declared the case closed and -- in a startling position for a scientific body -- recommended that no further research be conducted.
The report may have satisfied the CDC, but it convinced no one. Rep. David Weldon, a Republican physician from Florida who serves on the House Government Reform Committee, attacked the Institute of Medicine, saying it relied on a handful of studies that were "fatally flawed" by "poor design" and failed to represent "all the available scientific and medical research." CDC officials are not interested in an honest search for the truth, Weldon told me, because "an association between vaccines and autism would force them to admit that their policies irreparably damaged thousands of children. Who would want to make that conclusion about themselves?"
Under pressure from Congress, parents and a few of its own panel members, the Institute of Medicine reluctantly convened a second panel to review the findings of the first. In February, the new panel, composed of different scientists, criticized the earlier panel for its lack of transparency and urged the CDC to make its vaccine database available to the public.
So far, though, only two scientists have managed to gain access. Dr. Mark Geier, president of the Genetics Center of America, and his son, David, spent a year battling to obtain the medical records from the CDC. Since August 2002, when members of Congress pressured the agency to turn over the data, the Geiers have completed six studies that demonstrate a powerful correlation between thimerosal and neurological damage in children. One study, which compares the cumulative dose of mercury received by children born between 1981 and 1985 with those born between 1990 and 1996, found a "very significant relationship" between autism and vaccines. Another study of educational performance found that kids who received higher doses of thimerosal in vaccines were nearly three times as likely to be diagnosed with autism and more than three times as likely to suffer from speech disorders and mental retardation. Another soon-to-be-published study shows that autism rates are in decline following the recent elimination of thimerosal from most vaccines.
As the federal government worked to prevent scientists from studying vaccines, others have stepped in to study the link to autism. In April, reporter Dan Olmsted of UPI undertook one of the more interesting studies himself. Searching for children who had not been exposed to mercury in vaccines -- the kind of population that scientists typically use as a "control" in experiments -- Olmsted scoured the Amish of Lancaster County, Penn., who refuse to immunize their infants. Given the national rate of autism, Olmsted calculated that there should be 130 autistics among the Amish. He found only four. One had been exposed to high levels of mercury from a power plant. The other three -- including one child adopted from outside the Amish community -- had received their vaccines.
At the state level, many officials have also conducted in-depth reviews of thimerosal. While the Institute of Medicine was busy whitewashing the risks, the Iowa Legislature was carefully combing through all of the available scientific and biological data. "After three years of review, I became convinced there was sufficient credible research to show a link between mercury and the increased incidences in autism," says state Sen. Ken Veenstra, a Republican who oversaw the investigation. "The fact that Iowa's 700 percent increase in autism began in the 1990s, right after more and more vaccines were added to the children's vaccine schedules, is solid evidence alone." Last year, Iowa became the first state to ban mercury in vaccines, followed by California. Similar bans are now under consideration in 32 other states.
But instead of following suit, the FDA continues to allow manufacturers to include thimerosal in scores of over-the-counter medications as well as steroids and injected collagen. Even more alarming, the government continues to ship vaccines preserved with thimerosal to developing countries -- some of which are now experiencing a sudden explosion in autism rates. In China, where the disease was virtually unknown prior to the introduction of thimerosal by U.S. drug manufacturers in 1999, news reports indicate that there are now more than 1.8 million autistics. Although reliable numbers are hard to come by, autistic disorders also appear to be soaring in India, Argentina, Nicaragua and other developing countries that are now using thimerosal-laced vaccines. The World Health Organization continues to insist thimerosal is safe, but it promises to keep the possibility that it is linked to neurological disorders "under review."
I devoted time to study this issue because I believe that this is a moral crisis that must be addressed. If, as the evidence suggests, our public-health authorities knowingly allowed the pharmaceutical industry to poison an entire generation of American children, their actions arguably constitute one of the biggest scandals in the annals of American medicine. "The CDC is guilty of incompetence and gross negligence," says Mark Blaxill, vice president of Safe Minds, a nonprofit organization concerned about the role of mercury in medicines. "The damage caused by vaccine exposure is massive. It's bigger than asbestos, bigger than tobacco, bigger than anything you've ever seen." It's hard to calculate the damage to our country -- and to the international efforts to eradicate epidemic diseases -- if Third World nations come to believe that America's most heralded foreign-aid initiative is poisoning their children. It's not difficult to predict how this scenario will be interpreted by America's enemies abroad. The scientists and researchers -- many of them sincere, even idealistic -- who are participating in efforts to hide the science on thimerosal claim that they are trying to advance the lofty goal of protecting children in developing nations from disease pandemics. They are badly misguided. Their failure to come clean on thimerosal will come back horribly to haunt our country and the world's poorest populations.
Robert F. Kennedy Jr. is senior attorney for the Natural Resources Defense Council, chief prosecuting attorney for Riverkeeper and president of Waterkeeper Alliance. He is the co-author of "The Riverkeepers."