Part 2 of 2
Materials and methods
Ethics statement
All sampling procedures were performed by veterinarians with approval from Animal Ethics Committee of the Wuhan Institute of Virology (WIVH05210201). The study was conducted in accordance with the Guide for the Care and Use of Wild Mammals in Research of the People’s Republic of China.
Sampling
Bat samplings were conducted ten times from April 2011 to October 2015 at different seasons in their natural habitat at a single location (cave) in Kunming, Yunnan Province, China. All members of field teams wore appropriate personal protective equipment, including N95 masks, tear-resistant gloves, disposable outerwear, and safety glasses. Bats were trapped and fecal swab samples were collected as described previously [9]. Clean plastic sheets measuring 2.0 by 2.0 m were placed under known bat roosting sites at about 18:00 h each evening for collection of fecal samples. Fresh fecal pellets were collected from sheets early in the next morning. Each sample (approximately 1 gram of fecal pellet) was collected in 1ml of viral transport medium composed of Hank's balanced salt solution at pH7.4 containing BSA (1%), amphotericin (15 μg/ml), penicillin G (100 units/ml), and streptomycin (50 μg/ml), and were stored at -80°C until processing. Bats trapped for this study were released back into their habitat.
RNA extraction, PCR screening and sequencing
Fecal swab or pellet samples were vortexed for 1 min, and 140 μl of supernatant was collected from each sample after centrifuge at 3000 rpm under 4°C for 1min. Viral RNA was extracted with Viral RNA Mini Kit (Qiagen) following the manufacturer’s instructions. RNA was eluted in 60 μl of buffer AVE (RNase-free water with 0.04% sodium azide, Qiagen), aliquoted, and stored at -80°C. One-step hemi-nested RT-PCR (Invitrogen) was employed to detect the presence of coronavirus sequences as described previously using a set of primers that target a 440-nt fragment in the RNA-dependent RNA polymerase gene (RdRp) of all known alpha- and betacoronaviruses [20]. For the first round PCR, the 25 μl reaction mix contained 12.5 μl PCR 2 × reaction mix buffer, 10 pmol of each primer, 2.5 mM MgSO4, 20 U RNase inhibitor, 1 μl SuperScript III/Platinum Taq Enzyme Mix and 5 μl RNA template. The amplification was performed as follows: 50°C for 30 min, 94°C for 2 min, followed by 40 cycles consisting of 94°C for 15 sec, 52°C for 30 sec, 68°C for 40 sec, and a final extension of 68°C for 5 min. For the second round PCR, the 25 μl reaction mix contained 2.5 μl PCR reaction buffer, 5 pmol of each primer, 50 mM MgCl2, 0.5mM dNTP, 0.1 μl Platinum Taq Enzyme (Invitrogen) and 1 μl product of the first round PCR. The amplification was performed as follows: 94°C for 3 min followed by 35 cycles consisting of 94°C for 30 sec, 52°C for 30 sec, 72°C for 40 sec, and a final extension of 72°C for 7 min. The RBD region was amplified using the one-step nested RT-PCR method previously described [17].
PCR products were gel purified and sequenced with an ABI Prism 3730 DNA analyzer (Applied Biosystems, USA). PCR products with low concentration or generating heterogeneity in the sequencing chromatograms were cloned into pGEM-T Easy Vector (Promega) for sequencing. The positive samples in this study were termed using the abbreviated name of bat species plus the sample ID number (e.g. Rs4081). To confirm the bat species of individual sample, PCR amplification of cytochrome b (Cytob) or NADH dehydrogenase subunit 1 (ND1) gene was performed using DNA extracted from the feces or swabs [38,39].
Sequencing of full-length genomes
Full genomic sequences of 11 SARSr-CoVs were determined by One-step PCR (Invitrogen) amplification of overlapping genomic fragments with degenerate primers designed by multiple alignment of available SARS-CoV and bat SARSr- CoV sequences deposited in GenBank, and additional specific primers designed from the results of previous rounds of sequencing in this study. Primer sequences are available upon request. Sequences of the 5’ and 3’ genomic ends were obtained by 5’ and 3’ RACE (Roche), respectively. PCR products with expected size were gel-purified and subjected directly to sequencing. Each fragment was sequenced at least twice. The sequencing chromatogram of each product was thoroughly examined and sequence heterogeneity was not observed. For some fragments with low concentration of amplicons, the PCR products were cloned into pGEM-T Easy Vector (Promega) for sequencing. At least five independent clones were sequenced to obtain a consensus sequence. Co-presence of sequences of distinct SARSr-CoVs was not found in any of the amplicons. The sequences of overlapping genomic fragments were assembled to obtain the full-length genome sequences, with each overlapping sequence longer than 100 bp.
Evolution analysis
Full-length genome sequences of the 15 SARSr-CoVs detected from bats in the cave surveyed in this study were aligned with those of selected SARS-CoVs using MUSCLE [40]. The aligned sequences were scanned for recombination events by Recombination Detection Program (RDP) [41]. The potential recombination events suggested by strong P values (<10−20) were further confirmed using similarity plot and bootscan analyses implemented in Simplot 3.5.1 [42]. Phylogenetic trees based on nucleotide sequences were constructed using the Maximum Likelihood algorithm under the LG model with bootstrap values determined by 1000 replicates in the PhyML (version 3.0) software package [43].
Virus isolation
The Vero E6 cell line was kindly provided by Australian Animal Health Laboratory, CSIRO (Geelong, Australia). Vero E6 monolayer was maintained in DMEM medium supplemented with 10% fetal calf serum (FCS). Fecal samples (in 200 μl buffer) were gradient centrifuged at 3,000–12,000 g, and the supernatant was diluted 1:10 in DMEM before being added to Vero E6 cells. After incubation at 37°C for 1 h, the inoculum was removed and replaced with fresh DMEM medium with 2% FCS. The cells were incubated at 37°C and checked daily for cytopathic effect. All tissue culture media were supplemented with triple antibiotics penicillin/ streptomycin/amphotericin (Gibco) (penicillin 200 IU/ml, streptomycin 0.2 mg/ml, amphotericin 0.5 μg/ml). Three blind passages were carried out for each sample. After each passage, both the culture supernatant and cell pellet were examined for presence of SARSr-CoV by RT-PCR using specific primers targeting the RdRp or S gene. The viruses which caused obvious cytopathic effect and could be detected in three blind passages by RT-PCR were further confirmed by electron microscopy.
Construction of recombinant viruses
Recombinant viruses with the S gene of the novel bat SARSr-CoVs and the backbone of the infectious clone of SARSr-CoV WIV1 were constructed using the reverse genetic system described previously [23] (S9 Fig). The fragments E and F were re-amplified with primer pairs (FE, 5’-AGGGCCCACCTGGCACTGGTAAGAGTCATTTTGC-3’, R-EsBsaI, 5’-ACTGGTCTCTTCGTTTAGTTATTAACTAAAATATCACTAGACACC-3’) and (F-FsBsaI, 5’-TGAGGTCTCCGAACTTATGGATTTGTTTATGAG-3’, RF, 5’-AGGTAGGCCTCTAGGGCAGCTAAC-3’), respectively. The products were named as fragment Es and Fs, which leave the spike gene coding region as an independent fragment. BsaI sites (5’-GGTCTCN|NNNN-3’) were introduced into the 3’ terminal of the Es fragment and the 5’ terminal of the Fs fragment, respectively. The spike sequence of Rs4231 was amplified with the primer pair (F-Rs4231-BsmBI, 5’-AGTCGTCTCAACGAACATGTTTATTTTCTTATTCTTTCTCACTCTCAC-3’ and R-Rs4231-BsmBI, 5’-TCACGTCTCAGTTCGTTTATGTGTAATGTAATTTGACACCCTTG-3’). The S gene sequence of Rs7327 was amplified with primer pair (F-Rs7327-BsaI, 5’-AGTGGTCTCAACGAACATGAAATTGTTAGTTTTAGTTTTTGCTAC-3’ and R-Rs7327-BsaI, 5’- TCAGGTCTCAGTTCGTTTATGTGTAATGTAATTTAACACCCTTG-3’). The fragment Es and Fs were both digested with BglI (NEB) and BsaI (NEB). The Rs4231 S gene was digested with BsmBI. The Rs7327 S gene was digested with BsaI. The other fragments and bacterial artificial chromosome (BAC) were prepared as described previously. Then the two prepared spike DNA fragments were separately inserted into BAC with Es, Fs and other fragments. The correct infectious BAC clones were screened. The chimeric viruses were rescued as described previously [23].
Determination of virus infectivity by immunofluorescence assay
The HeLa cell line was kindly provided by Australian Animal Health Laboratory, CSIRO (Geelong, Australia). HeLa cells expressing human ACE2 were constructed as described previously [17]. HeLa cells expressing human ACE2 and Vero E6 cells were cultured on coverslips in 24-well plates (Corning) incubated with the newly isolated or recombinant bat SARSr-CoVs at a multiplicity of infection (MOI) = 1.0 for 1h. The inoculum was removed and the cells were washed twice with PBS and supplemented with medium. Vero E6 cells without virus inoculation and HeLa cells without ACE2 were used as negative control. Twenty-four hours after infection, cells were rinsed with PBS and fixed with 4% formaldehyde in PBS (pH7.4) at 4°C for 20 min. ACE2 expression was detected by using goat anti-human ACE2 immunoglobulin followed by FITC-labelled donkey anti-goat immunoglobulin (PTGLab). Virus replication was detected by using rabbit antibody against the nucleocapsid protein of bat SARSr-CoV Rp3 followed by Cy3-conjugated mouse anti-rabbit IgG. Nuclei were stained with DAPI. Staining patterns were observed under an FV1200 confocal microscope (Olympus).
Determination of virus replication in Vero E6 cells by plaque assay
Vero E6 cells were infected with WIV1, Rs4874, WIV1-Rs4231S, and WIV1-Rs7327S at an MOI of 1.0 and 0.01. After incubation for an hour, the cells were washed with DHanks for three times and supplied with DMEM containing 2% FCS. Samples were collected at 0, 10, 27, and 48 h post infection. The viral titers were determined by plaque assay.
Determination of virus replication in HeLa cells expressing human ACE2 by quantitative RT-PCR
HeLa cells expressing human ACE2 were inoculated with WIV1, Rs4874, WIV1-Rs4231S, and WIV1-Rs7327S at an MOI of 1.0, and were incubated for 1h at 37°C. After the inoculum was removed, the cells were supplemented with medium containing 1% FBS. Supernatants were collected at 0, 12, 24 and 48h. Virus titers were determined using quantitative RT-PCR targeting the partial N gene with a standard curve which expresses the correlation between Ct value and virus titer (shown as TCID50/ml). The standard curve was made using RNA dilutions from the purified Rs4874 virus stock (with a titer of 2.15 × 106 TCID50/ml). For qPCR, RNA was extracted from 140 μl of each supernatant with Viral RNA Mini Kit (Qiagen) following manufacturer’s instructions and eluted in 60 μl AVE buffer. The PCR was performed with the TaqMan AgPath-ID One-Step RT–PCR Kit (Applied Biosystems) in a 25 μl reaction mix containing 4 μl RNA, 1 × RT–PCR enzyme mix, 1 × RT–PCR buffer, 40 pmol forward primer (5’-GTGGTGGTGACGGCA AAATG-3’), 40 pmol reverse primer (5’-AAGTGAAGCTTCTGGGCCAG-3’) and 12 pmol probe (5’-FAM-AAAGAGCTCAGCCCCAGATG-BHQ1-3’). The amplification was performed as follows: 50°C for 10 min, 95°C for 10 min followed by 50 cycles consisting of 95°C for 15 sec and 60°C for 20 sec.
Plasmids
The ORF8 genes of bat SARSr-CoV WIV1 and Rf4092 and the ORF8a gene of bat SARSr-CoV Rs4084 were amplified by PCR from the viral RNA extracted from the isolated virus or fecal samples. The ORF8 gene of SARS-CoV GZ02 and bat SARSr-CoV Rf1, and the ORF8a gene of SARS-CoV Tor2 were synthesized by Tsingke Biological Technology Co., Ltd (Wuhan, China). All genes were cloned into the pCAGGS vector constructed with a C-terminal HA tag. Expression of the proteins was confirmed by Western blotting using a mAb against the HA tag. Five tandem copies of the ATF6 consensus binding sites were synthesized and inserted into the pGL3-Basic vector to construct the luciferase reporter plasmid 5×ATF6-GL3, in which the luciferase gene is under the control of the c-fos minimal promoter and the ATF6 consensus binding sites.
Luciferase reporter assay
HeLa cells in 24-well plates were transfected using Lipofectamine 3000 reagent (Life Technologies) following the manufacturer’s instruction. Cells per well were co-transfected with 600ng of the 5×ATF6-GL3 reporter plasmid, with 300ng of each expression plasmid of SARS-CoV and SARSr-CoV ORF8 or empty vector and 20ng of pRL-TK (Promega) which served as an internal control. The cells were incubated for 24h, and were treated with or without 2μg/ml tunicamycin for 16h. Cells were harvested and lysed. Luciferase activity was determined using a dual-luciferase assay system (Promega). The experiment was performed in triplicate wells.
Quantification of apoptotic cells
293T cells in 12-well plates were transfected using Lipofectamine 3000 reagent (Life Technologies) following the manufacturer’s instruction. Cells per well were transfected with 3μg of the expression plasmid of SARS-CoV Tor2 or SARSr-CoV Rs4084 ORF8a, or the empty vector. 24h post transfection, apoptotic cells were quantified by using the Annexin V-fluorescein isothiocyanate (FITC)/PI Apoptosis Detection Kit (Yeasen Biotech, Shanghai) in accordance with the manufacturer’s instruction. Apoptosis was analyzed by flow cytometry. The experiment was performed in triplicate wells.
Accession numbers
The complete genome sequences of bat SARS-related coronavirus strains As6526, Rs4081, Rs4084, Rf4092, Rs4231, Rs4237, Rs4247, Rs4255, Rs4874, Rs7327 and Rs9401 have been deposited in the GenBank database with the accession numbers from KY417142 to KY417152, respectively.
Supporting information
S1 Fig
Alignment of amino acid sequences of the receptor-binding motif (corresponding to aa 424–495 of SARS-CoV S protein).
Two clades of the SARSr-CoVs identified from bats in the studied cave are indicated with vertical lines on the left.
(PPTX)
Click here for additional data file.(94K, pptx)
S2 Fig
Alignment of nucleotide sequences of a genomic region covering ORF6 to ORF7a.
ORFX is located between ORF6 and ORF7a in the genomes of WIV1, WIV16, Rs7327 and Rs4874. The start codon and stop codon of ORFX are marked with red boxes. The deletion responsible for the long ORFX in Rs7327 and Rs4874 is marked with the blue box.
(PPTX)
Click here for additional data file.(165K, pptx)
S3 Fig
Phylogenetic analyses based on nucleotide sequences of the S gene (A), ORF3a (B) and ORF8 (C). The trees were constructed by the maximum likelihood method using the LG model with bootstrap values determined by 1000 replicates. Only bootstraps > 50% are shown. Rs, Rhinolophus sinicus; Rf, Rhinolophus ferremequinum; Rm, Rhinolophus macrotis; Ra, Rhinolophus affinis; Rp, Rhinolophus pusillus; As, Aselliscus stoliczkanus; Cp, Chaerephon plicata. SARSr-CoVs detected in bats from the single cave surveyed in this study are in bold.
(PPTX)
Click here for additional data file.(1.7M, pptx)
S4 Fig
Alignment of amino acid sequences of ORF3b protein.
(PPTX)
Click here for additional data file.(144K, pptx)
S5 Fig
Detection of potential recombination events by similarity plot and boot scan analysis.
(A) Full-length genome sequence of SARSr-CoV Rs4084 was used as query sequence and RsSHC014, Rf4092 and Rs4081 as reference sequences. (B) Full-length genome sequence of SARSr-CoV Rs4237 was used as query sequence and SARSr-CoV Rs4247, Rs4081 and Rs3367 as reference sequences. All analyses were performed with a Kimura model, a window size of 1500 base pairs, and a step size of 150 base pairs.
(PPTX)
Click here for additional data file.(850K, pptx)
S6 Fig
Chinese provinces where bat SARSr-CoVs have been detected.
(PPTX)
Click here for additional data file.(83K, pptx)
S7 Fig
The successful or failed rescue of the chimeric SARSr-CoVs.
(A) Cytopathic effects in Vero E6 cells transfected with the infectious BAC clones constructed with the backbone of WIV1 and various S genes of different bat SARSr-CoV strains. Microphotographs were taken 24 hours post transfection. (B) The culture media supernatant collected from the cells transfected with the infectious BAC clones was used to infect Vero E6 cells. Immunofluorescent assay (IFA) was performed to detect infection and viral replication. Cells were fixed 24 hours post infection, and stained using rabbit antibody against the SARSr-CoV Rp3 nucleocapsid protein and a Cy3-conjugated anti-rabbit IgG.
(PPTX)
Click here for additional data file.(8.3M, pptx)
S8 Fig
Quantification of SARSr-CoV in individual bat fecal samples.
The number of genome copies of SARSr-CoV per gram of bat feces was determined by quantitative real-time PCR targeting the RdRp gene. Samples from which the SARSr-CoV RBD sequences were successfully amplified are indicated in red.
(PPTX)
Click here for additional data file.(374K, pptx)
S9 Fig
Spike substitution strategy.
The original fragments E and F were shortened to leave spike gene as an independent fragment. The new fragments were designated as Es and Fs. BsaI or BsmBI sites were introduced into the junctions of Es/Spike and Spike/Fs. Then any spike could be substituted into the genome of SARSr-CoV WIV1 through this strategy.
(TIF)
Click here for additional data file.(1.3M, tif)
S1 Table
Comparison of the novel bat SARSr-CoVs identified in this study with human/civet SARS-CoVs and previously described bat SARSr-CoVs.
(DOCX)
Click here for additional data file.(36K, docx)
S2 Table
Distribution of SARSr-CoVs highly similar to SARS-CoV in the variable S, ORF3 and ORF8 genes in the single cave.
(DOCX)
Click here for additional data file.(15K, docx)
S1 Dataset
Full-length genome sequences of bat SARSr-CoVs newly identified in this study.
(FAS)
Click here for additional data file.(326K, fas)
Acknowledgments
We thank Ji-Hua Zhou and Wei-Hong Yang from Yunnan Institute of Endemic Diseases Control and Prevention for the assistance in sample collection. We thank the Center for Instrumental Analysis and Metrology of Wuhan Institute of Virology, CAS, for the assistance in taking confocal microscope pictures (Dr. Ding Gao) and flow cytometry (Ms. Juan Min).
Funding Statement
This work was jointly funded by National Natural Science Foundation of China (81290341, 31621061) to ZLS, China Mega-Project for Infectious Disease (2014ZX10004001-003) to ZLS, Scientific and technological basis special project (2013FY113500) to YZZ and ZLS from the Ministry of Science and Technology of China, the Strategic Priority Research Program of the Chinese Academy of Sciences (XDPB0301) to ZLS, the National Institutes of Health (NIAID R01AI110964), the USAID Emerging Pandemic Threats (EPT) PREDICT program to PD and ZLS, CAS Pioneer Hundred Talents Program to JC, NRF-CRP grant (NRF-CRP10-2012-05) to LFW and WIV “One-Three-Five” Strategic Program (WIV-135-TP1) to JC and ZLS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files. The complete genome sequences of the 11 bat SARS-related coronaviruses newly identified in this study have been deposited in the GenBank database and assigned accession numbers KY417142 to KY417152, respectively.
References
1. Peiris JS, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med. 2004; 10: S88–97. doi: 10.1038/nm1143 [PMC free article] [PubMed] [Google Scholar]
2. Zhong NS, Zheng BJ, Li YM, Poon, Xie ZH, Chan KH, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003; 362: 1353–1358. [PMC free article] [PubMed] [Google Scholar]
3. Chinese SMEC. Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004; 303: 1666–1669. doi: 10.1126/science.1092002 [PubMed] [Google Scholar]
4. Drexler JF, Corman VM, Drosten C. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral Res. 2014; 101: 45–56. doi: 10.1016/j.antiviral.2013.10.013 [PMC free article] [PubMed] [Google Scholar]
5. Marra MA, Jones SJ, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YS, et al. The Genome sequence of the SARS-associated coronavirus. Science. 2003; 300: 1399–1404. doi: 10.1126/science.1085953 [PubMed] [Google Scholar]
6. Snijder EJ, Bredenbeek PJ, Dobbe JC, Thiel V, Ziebuhr J, Poon LL, et al. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J Mol Biol. 2003; 331: 991–1004. [PMC free article] [PubMed] [Google Scholar]
7. Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003; 302: 276–278. doi: 10.1126/science.1087139 [PubMed] [Google Scholar]
8. Song HD, Tu CC, Zhang GW, Wang SY, Zheng K, Lei LC, et al. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc Natl Acad Sci U S A. 2005; 102: 2430–2435. doi: 10.1073/pnas.0409608102 [PMC free article] [PubMed] [Google Scholar]
9. Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005; 310: 676–679. doi: 10.1126/science.1118391 [PubMed] [Google Scholar]
10. Lau SK, Woo PC, Li KS, Huang Y, Tsoi HW, Wong BH, et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci U S A. 2005; 102: 14040–14045. doi: 10.1073/pnas.0506735102 [PMC free article] [PubMed] [Google Scholar]
11. Tang XC, Zhang JX, Zhang SY, Wang P, Fan XH, Li LF, et al. Prevalence and genetic diversity of coronaviruses in bats from China. J Virol. 2006; 80: 7481–7490. doi: 10.1128/JVI.00697-06 [PMC free article] [PubMed] [Google Scholar]
12. Yuan J, Hon CC, Li Y, Wang D, Xu G, Zhang H, et al. Intraspecies diversity of SARS-like coronaviruses in Rhinolophus sinicus and its implications for the origin of SARS coronaviruses in humans. J Gen Virol. 2010; 91: 1058–1062. doi: 10.1099/vir.0.016378-0 [PubMed] [Google Scholar]
13. He B, Zhang Y, Xu L, Yang W, Yang F, Feng Y, et al. Identification of diverse alphacoronaviruses and genomic characterization of a novel severe acute respiratory syndrome-like coronavirus from bats in China. J Virol. 2014; 88: 7070–7082. doi: 10.1128/JVI.00631-14 [PMC free article] [PubMed] [Google Scholar]
14. Wu Z, Yang L, Ren X, He G, Zhang J, Yang J, et al. Deciphering the bat virome catalog to better understand the ecological diversity of bat viruses and the bat origin of emerging infectious diseases. ISME J. 2016; 10: 609–620. doi: 10.1038/ismej.2015.138 [PMC free article] [PubMed] [Google Scholar]
15. Drexler JF, Gloza-Rausch F, Glende J, Corman VM, Muth D, Goettsche M, et al. Genomic characterization of severe acute respiratory syndrome-related coronavirus in European bats and classification of coronaviruses based on partial RNA-dependent RNA polymerase gene sequences. J Virol. 2010; 84: 11336–11349. doi: 10.1128/JVI.00650-10 [PMC free article] [PubMed] [Google Scholar]
16. Tong S, Conrardy C, Ruone S, Kuzmin IV, Guo X, Tao Y, et al. Detection of novel SARS-like and other coronaviruses in bats from Kenya. Emerg Infect Dis. 2009; 15: 482–485. doi: 10.3201/eid1503.081013 [PMC free article] [PubMed] [Google Scholar]
17. Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013; 503: 535–538. doi: 10.1038/nature12711 [PMC free article] [PubMed] [Google Scholar]
18. Yang XL, Hu B, Wang B, Wang MN, Zhang Q, Zhang W, et al. Isolation and Characterization of a Novel Bat Coronavirus Closely Related to the Direct Progenitor of Severe Acute Respiratory Syndrome Coronavirus. J Virol. 2016; 90: 3253–3256. [PMC free article] [PubMed] [Google Scholar]
19. Menachery VD, Yount BL Jr., Sims AC, Debbink K, Agnihothram SS, Gralinski LE, et al. SARS-like WIV1-CoV poised for human emergence. Proc Natl Acad Sci U S A. 2016; 113: 3048–3053. doi: 10.1073/pnas.1517719113 [PMC free article] [PubMed] [Google Scholar]
20. de Souza Luna LK, Heiser V, Regamey N, Panning M, Drexler JF, Mulangu S, et al. Generic detection of coronaviruses and differentiation at the prototype strain level by reverse transcription-PCR and nonfluorescent low-density microarray. J Clin Microbiol. 2007; 45: 1049–1052. doi: 10.1128/JCM.02426-06 [PMC free article] [PubMed] [Google Scholar]
21. Ren W, Li W, Yu M, Hao P, Zhang Y, Zhou P, et al. Full-length genome sequences of two SARS-like coronaviruses in horseshoe bats and genetic variation analysis. J Gen Virol. 2006; 87: 3355–3359. doi: 10.1099/vir.0.82220-0 [PubMed] [Google Scholar]
22. Lau SK, Feng Y, Chen H, Luk HK, Yang WH, Li KS, et al. Severe Acute Respiratory Syndrome (SARS) Coronavirus ORF8 Protein Is Acquired from SARS-Related Coronavirus from Greater Horseshoe Bats through Recombination. J Virol. 2015; 89: 10532–10547. doi: 10.1128/JVI.01048-15 [PMC free article] [PubMed] [Google Scholar]
23. Zeng LP, Gao YT, Ge XY, Zhang Q, Peng C, Yang XL, et al. Bat Severe Acute Respiratory Syndrome-Like Coronavirus WIV1 Encodes an Extra Accessory Protein, ORFX, Involved in Modulation of the Host Immune Response. J Virol. 2016; 90: 6573–6582. doi: 10.1128/JVI.03079-15 [PMC free article] [PubMed] [Google Scholar]
24. Li F. Evidence for a common evolutionary origin of coronavirus spike protein receptor-binding subunits. J Virol. 2012; 86: 2856–2858. doi: 10.1128/JVI.06882-11 [PMC free article] [PubMed] [Google Scholar]
25. Lau SK, Li KS, Huang Y, Shek CT, Tse H, Wang M, et al. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J Virol. 2010; 84: 2808–2819. doi: 10.1128/JVI.02219-09 [PMC free article] [PubMed] [Google Scholar]
26. Oostra M, de Haan CA, Rottier PJ. The 29-nucleotide deletion present in human but not in animal severe acute respiratory syndrome coronaviruses disrupts the functional expression of open reading frame 8. J Virol. 2007; 81: 13876–13888. doi: 10.1128/JVI.01631-07 [PMC free article] [PubMed] [Google Scholar]
27. Kopecky-Bromberg SA, Martinez-Sobrido L, Frieman M, Baric RA, Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J Virol. 2007; 81: 548–557. doi: 10.1128/JVI.01782-06 [PMC free article] [PubMed] [Google Scholar]
28. Chen CY, Ping YH, Lee HC, Chen KH, Lee YM, Chen YJ, et al. Open reading frame 8a of the human severe acute respiratory syndrome coronavirus not only promotes viral replication but also induces apoptosis. J Infect Dis. 2007; 196: 405–415. doi: 10.1086/519166 [PMC free article] [PubMed] [Google Scholar]
29. Yang L, Wu Z, Ren X, Yang F, He G, Zhang J, et al. Novel SARS-like betacoronaviruses in bats, China, 2011. Emerg Infect Dis. 2013; 19: 989–991. doi: 10.3201/eid1906.121648 [PMC free article] [PubMed] [Google Scholar]
30. Wu Z, Yang L, Ren X, Zhang J, Yang F, Zhang S, et al. ORF8-Related Genetic Evidence for Chinese Horseshoe Bats as the Source of Human Severe Acute Respiratory Syndrome Coronavirus. J Infect Dis. 2016; 213: 579–583. doi: 10.1093/infdis/jiv476 [PMC free article] [PubMed] [Google Scholar]
31. Drexler JF, Corman VM, Wegner T, Tateno AF, Zerbinati RM, Gloza-Rausch F, et al. Amplification of emerging viruses in a bat colony. Emerg Infect Dis. 2011; 17: 449–456. doi: 10.3201/eid1703.100526 [PMC free article] [PubMed] [Google Scholar]
32. Wang MN, Zhang W, Gao YT, Hu B, Ge XY, Yang XL, et al. Longitudinal surveillance of SARS-like coronaviruses in bats by quantitative real-time PCR. Virol Sin. 2016; 31: 78–80. doi: 10.1007/s12250-015-3703-3 [PMC free article] [PubMed] [Google Scholar]
33. Menachery VD, Yount BL Jr., Debbink K, Agnihothram S, Gralinski LE, Plante JA, et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med. 2015; 21: 1508–1513. doi: 10.1038/nm.3985 [PMC free article] [PubMed] [Google Scholar]
34. Ren W, Qu X, Li W, Han Z, Yu M, Zhou P, et al. Difference in receptor usage between severe acute respiratory syndrome (SARS) coronavirus and SARS-like coronavirus of bat origin. J Virol. 2008; 82: 1899–1907. doi: 10.1128/JVI.01085-07 [PMC free article] [PubMed] [Google Scholar]
35. Sung SC, Chao CY, Jeng KS, Yang JY, Lai MM. The 8ab protein of SARS-CoV is a luminal ER membrane-associated protein and induces the activation of ATF6. Virology. 2009; 387: 402–413. doi: 10.1016/j.virol.2009.02.021 [PMC free article] [PubMed] [Google Scholar]
36. Freundt EC, Yu L, Park E, Lenardo MJ, Xu XN. Molecular determinants for subcellular localization of the severe acute respiratory syndrome coronavirus open reading frame 3b protein. J Virol. 2009; 83: 6631–6640. doi: 10.1128/JVI.00367-09 [PMC free article] [PubMed] [Google Scholar]
37. Zhou P, Li H, Wang H, Wang LF, Shi Z. Bat severe acute respiratory syndrome-like coronavirus ORF3b homologues display different interferon antagonist activities. J Gen Virol. 2012; 93: 275–281. doi: 10.1099/vir.0.033589-0 [PubMed] [Google Scholar]
38. Irwin DM, Kocher TD, Wilson AC. Evolution of the cytochrome b gene of mammals. J Mol Evol. 1991; 32: 128–144. [PubMed] [Google Scholar]
39. Mayer F, von Helversen O. Cryptic diversity in European bats. Proc Biol Sci. 2001; 268: 1825–1832. doi: 10.1098/rspb.2001.1744 [PMC free article] [PubMed] [Google Scholar]
40. Hall BG. Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol. 2013; 30: 1229–1235. doi: 10.1093/molbev/mst012 [PubMed] [Google Scholar]
41. Martin DP, Lemey P, Lott M, Moulton V, Posada D, Lefeuvre P. RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics. 2010; 26: 2462–2463. doi: 10.1093/bioinformatics/btq467 [PMC free article] [PubMed] [Google Scholar]
42. Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999; 73: 152–160. [PMC free article] [PubMed] [Google Scholar]
43. Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010; 59: 307–321. doi: 10.1093/sysbio/syq010 [PubMed] [Google Scholar]
U.S. government gave $3.7 million grant to Wuhan lab at cent
Re: U.S. government gave $3.7 million grant to Wuhan lab at
Part 1 of 3
Lab-Made? SARS-CoV-2 Genealogy Through the Lens of Gain-of-Function Research
by Yuri Deigin
Apr 22, 2020
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT

Staff celebrating the physical completion of the laboratory in 2015, Wuhan, China (Source)
How I Learned to Start Worrying
Oh, come on. Lab-made? Nonsense! Back in January, that was my knee-jerk reaction when ideas that Covid-19 is caused by a laboratory leak had just surfaced. Bioweapon? Well, that is just Flat Earth crazies territory. Thus, whenever I kept hearing anything about non-natural origins of SARS-CoV-2, I brushed it aside under similar sentiments. So what if there is a virology institute in Wuhan? Who knows how many of those are sprinkled throughout China.
At some point, it became necessary to brush such theories aside in a substantiated manner, as their proponents began to back up their theses about the possible artificial nature of the virus with arguments from molecular biology, and when engaging them in debate, I wanted to smash their conspiracy theories with cold, hard scientific facts. Just like that Nature paper (or so I thought).
So it was then, in pursuit of arguments against the virus’s lab-madeness, that I got infected by the virus of doubt. What was the source of my doubts? The fact that the deeper you dive into the research activities of coronavirologists over the past 15–20 years, the more you realize that creating chimeras like CoV2 was commonplace in their labs.
And CoV2 is an obvious chimera (though not necessarily a lab-made one), which is based on the ancestral bat strain RaTG13, in which the receptor binding motif (RBM) in its spike protein is replaced by the RBM from a pangolin strain, and in addition, a small but very special stretch of 4 amino acids is inserted, which creates a furin cleavage site that, as virologists have previously established, significantly expands the “repertoire” of the virus in terms of whose cells it can penetrate. Most likely, it was thanks to this new furin site that the new mutant managed to jump species from its original host to humans.
Indeed, virologists, including the leader of coronavirus research at the Wuhan Institute of Virology, Shi Zhengli, have done many similar things in the past — both replacing the RBM in one type of virus by an RBM from another, or adding a new furin site that can provide a species-specific coronavirus with an ability to start using the same receptor (e.g. ACE2) in other species. In fact, Shi Zhengli’s group was creating chimeric constructs as far back as 2007 and as recently as 2017, when they created a whole of 8 new chimeric coronaviruses with various RBMs. In 2019 such work was in full swing, as WIV was part of a $3.7 million NIH grant titled Understanding the Risk of Bat Coronavirus Emergence. Under its auspices, Shi Zhengli co-authored a 2019 paper that called for continued research into synthetic viruses and testing them in vitro and in vivo:
If the above quote might seem vague as to what exactly “using reverse genetics” might mean, the NIH grant itself spells it out:
“Infectious clone technology” stands for creating live synthetic viral clones. Considering the heights of user friendliness and automation that genetic engineering tools have attained, creating a synthetic CoV2 via the above methodology would be in reach of even a grad student.
But before delving into CoV2 origins, let’s first take a quick dive into its biology.
Biology
Ok, let’s start from the basics. What’s a furin site, an RBM, or a spike protein? Bear with me: once you wade through the jungle of terminology, conceptually, everything is pretty straightforward. For example, spike proteins are those red things sticking out of a virus particle — the very reason for which these viruses got “crowned”:
It is with the help of these proteins that the virion clings to the receptor of the victim cell (ACE2 in our case) to then penetrate inside. So it is a vitally important part of the virus, as without getting into a cell viruses cannot replicate. The spike protein also determines which animals the virus can or cannot infect, as ACE2 receptors (or other targets for other viruses) in different species can differ in structure. At the same time, out of the entire 30 kilobase genome (quite huge by viral standards), the gene of this protein makes up only 12–13%. So the spike protein is only about 1300 amino acids long. Below is how the spike (S) protein is structured in CoV2 and close relatives:
As can be seen from the figure above, the S protein consists of two subunits: S1 and S2. It is S1 that interacts with the ACE2 receptor, and the place where S1 does so is called Receptor Binding Domain (RBD), while the area of direct contact, the holy of holies, is called Receptor Binding Motif (RBM). Here is a beautiful illustration from an equally beautiful work:
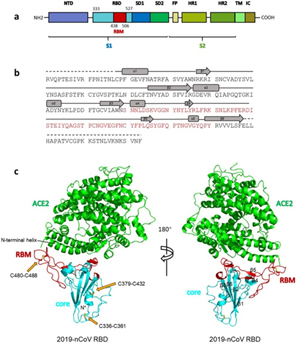
Overall structure of 2019-nCoV RBD bound with ACE2.
(a) Overall topology of 2019-nCoV spike monomer. NTD, N-terminal domain. RBD, receptor-binding domain. RBM, receptor-binding motif. SD1, subdomain 1. SD2, subdomain 2. FP, fusion peptide. HR1, heptad repeat 1. HR2, heptad repeat 2. TM, transmembrane region. IC, intracellular domain.
(b) Sequence and secondary structures of 2019-nCoV RBD. The RBM is colored red.
© Overall structure of 2019-nCoV RBD bound with ACE2. ACE2 is colored green. 2019-nCoV RBD core is colored cyan and RBM is colored red. Disulfide bonds in the 2019-nCoV RBD are shown as stick and indicated by yellow arrows. The N-terminal helix of ACE2 responsible for binding is labeled.
When the CoV2 genome was just sequenced and made publicly available on January 10, 2020, it was a riddle, as no closely related strains were known. But quite quickly, on January 23, Shi Zhengli released a paper indicating that CoV2 is 96% identical to RaTG13, a strain which her laboratory had previously isolated from Yunnan bats in 2013. However, outside of her lab, no one knew about that strain until January 2020.
It was immediately clear that RaTG13 is special. Take a look at the figure below:
This is a genome similarity graph between CoV2 and other known strains. The higher the curve, the higher the percentage of matching nucleotides. As you can see, in the spike protein (S) gene region (between nucleotides 22k and 25k), only RaTG13 is more or less close to CoV2, while all other strains take a deep dive around this spot — both strains from other bats and the first SARS-CoV (red curve). This in itself is far from suspicious — who knows how many unknown SARS-like strains lurk in the bat caves of Yunnan? Ok, maybe it is not very clear how exactly the virus could get from there to Wuhan, but hey, with those wet markets you never know.
Pangolins
Next, pangolins appeared on the scene: in February, another group of Chinese scientists discovered a peculiar strain of pangolin coronavirus in their possession, which, while generally being only 90% similar to CoV2, in the RBM region was almost identical to it, with only a single amino acid difference (see the upper two sequences, dots indicate a match with the top sequence):

Surprisingly, in the first quarter of the S protein, the pangolin strain is highly dissimilar from CoV2, but after the RBM all three strains (CoV2, Pangolin, RaTG13) exhibit a shared high degree of similarity. Most strikingly, RaTG13’s RBM itself is quite different than that of CoV2, which can be seen from the steep dive of the green RaTG13 graph compared to the red CoV2 graph in the RBM region (pink strip) in the following graph:
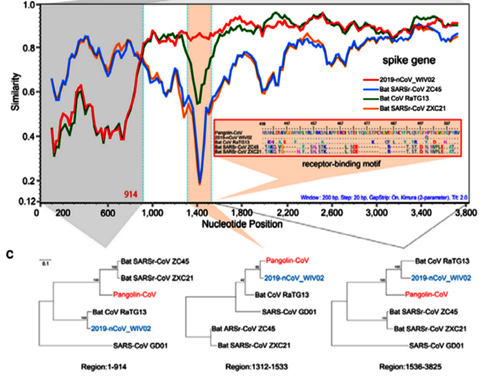
This observation is confirmed by the phylogenetic analysis of the three areas highlighted in the graph above — in the RBM, the pangolin strain is closer to CoV2 than is RaTG13, but it is RaTG13 that is closer to CoV2 to the left and right of RBM. So there is obvious recombination, as the authors (and other papers) conclude.
How did the researchers obtain those pangolins? This is how:

They were confiscated from smugglers by Chinese customs and transferred to an animal rehab center in Guangdong, where they died while exhibiting severe coronavirus symptoms. This, of course, must have gotten the attention of local virologists, who took several samples:
Those pangolins attracted the attention of other virologists too. For example, a team in Hong Kong also received samples of confiscated pangolins and in February 2020 they also released a paper that noted clear signs of recombination in the CoV2 spike protein:
By the way, the authors of this article also highlighted the high phylogenetic mosaicity of the CoV2 spike protein:
Translated from science-speak, what this means is that if we analyze the entire RBD of the three strains, ignoring the obvious differences (i.e. non-synonymous substitutions) among them, which are mainly found in the RBM (which, recall, is identical between CoV2 and Pangolin), and construct a phylogenetic tree for synonymous substitutions, CoV2 is still closer to RaTG13 than to the pangolin strain. Which is rather strange in light of the fact that the pangolin strain and CoV2 have identical RBMs (which are segments inside RBD).
The authors go on to put forth a conjecture that this may be the result of convergent evolution, in other words, that CoV2 and the pangolin strain came to possess identical RBMs each in their own way, rather than through recombination between common ancestors. Because it would have required a rather unique recombination event — as if someone cut out a precise RBM segment from a pangolin strain and used it to replace the RBM in RaTG13. Talk about Intelligent Design!
Royal Genealogy
In order to better understand CoV2 origins, let’s take a look at spike protein sequences of our Unholy Trinity: CoV2, RaTG13 and MP789 (pangolin-2019). Let’s compare the pairwise differences between them (identical amino acids are marked with dots, red letters denote differences, and dashes indicate deleted/inserted amino acids):
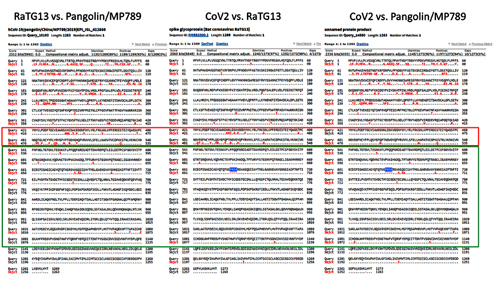
The comparisons illustrate what previously quoted papers have noted: that in the first quarter of the sequence, the pangolin strain is far from CoV2 and RaTG1, and if it weren’t for the RBM region (red rectangle), RaTG13 would have been very close to CoV2. But, as I already said, the RBM in CoV2 is closest to that of the pangolin strain.
What about other pangolin strains? So far we’ve only analyzed the MP789 strain isolated from pangolins confiscated by customs in 2019. But there was another batch of pangolins confiscated in 2017, and they also had a similar coronavirus strain isolated. Let’s compare it to RaTG13 and MP789:
In the first quarter of the S protein, the 2017 pangolin strains are closer to RaTG13 (and CoV2) than their 2019 pangolin counterpart (MP789). At the same time, all three have a clear recent common ancestor in the areas marked by green rectangles, and in these areas RaTG13 and pangolin-2019 (MP789) are closer to each other than to pangolin-2017, since they have several common mutations (marked by red and blue ellipses), which are absent from pangolin-2017. But the RBM for all three is different, and different in approximately the same proportion, and in similar places.
Maybe after ancestors of RaTG13 and MP789 diverged, the MP789 ancestor had the first quarter of its protein replaced (which did not occur in RaTG13 or pangolin-2017), and the rest of the protein remained common for all three strains. Later the paths of the RaTG13 and MP789 gene pools crossed again and produced CoV2. It is also possible that the ancestor of RaTG13 arose as a result of recombination of ancestral pangolin strains.
It is also interesting to see a rather unique identical mutation (QTQTNS) in RaTG13 and pangolin-2019 right in front of the spot where CoV2 has a new furin cleavage site. That furin site, as I mentioned, arose via an insertion of 4 new amino acids (PRRA). If we look at the nucleotide sequence around this insertion, we can see that RaTG13 and CoV2 are closer to each other in that area than to pangolin-2019, since they possess several common mutations (highlighted in blue):

By the way, Orf1ab is also a phylogenetic mess in CoV2: 1a is closer to RaTG13, but 1b is closer to pangolin-2019:
(Image Source)
Does this mean that the ancestor of CoV2 crossed with the common ancestor of pangolin-19 at least twice? First, when it (along with a common ancestor of RaTG13) inherited Orf1ab and the second half of the spike protein with the QTQTNS mutation, and second time when it acquired 1b and RBM, which differ from RaTG13. All of this is certainly possible in nature — after all, these viruses mutate and recombine constantly. Another question is where exactly bat and pangolin viruses are most likely to encounter one another for such orgies — in mountain caves, “wet markets”, shelters for confiscated animals, or even in laboratories. But let’s put those questions aside for now. First, let's discuss what is arguably the most eye-catching aspect of the new virus — a 4-amino acid insertion that turned it into a natural-born killer.
A Killer Intro
It is impossible to ignore the introduction of a PRRA insert between S1 and S2: it sticks out like a splinter. This insert creates the furin cleavage site, which I mentioned at the very beginning. Let me explain what a furin site is. Remember the structure of our spike protein? Here is a detailed diagram:
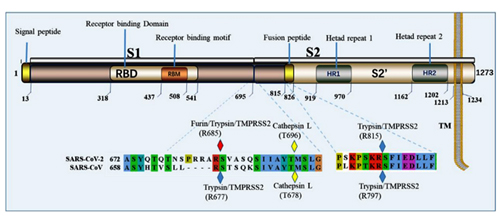
The protein consists of two parts, S1 and S2, of which S1 is responsible for primary contact with the receptor (recall Receptor Binding Domain / Motif), and S2 is responsible for fusion with the cell membrane and penetration into the cell. The fusion process is started by the fusion peptide marked in yellow, but in order for it to engage in its dirty deed, someone must cut the S protein at one of the sites marked by diamonds in the diagram above. The virus does not have its own such “cutters”, so it relies on various proteases of its victims. There are several types of such proteases, as can be deduced from the abundance of colors of those diamonds. But not all proteases are equal, and not all types of cells have proteases needed by the virus. Furin is one of the most effective, and it is found not only on the surface of cells, but also inside. Most clearly, the danger of the new furin site is demonstrated by the difference between CoV2 and its grandpa, SARS-CoV:
As can be seen from the diagram, in the case of CoV2, thanks to the furin site, it is not two, but three classes of proteases (three colored PacMans) that can cut its S protein outside the cell. But perhaps the most important difference is that furin is also present inside the cell, so it can cut the S protein immediately after virion assembly, thereby providing new virions with the ability to merge with new cells right off the bat (no pun intended).
The importance of the new furin site in CoV2’s virulence was recently demonstrated by a study in hamsters where the disappearance of the furin site (due to a mutation) greatly decreased mutant CoV2’s pathogenicity and replication ability:
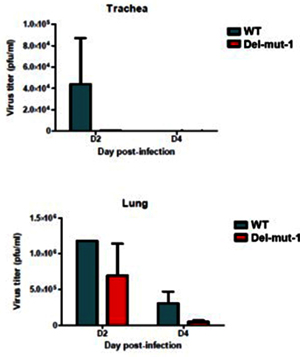
Virus replication in the lung tissues of hamsters infected with either WT or Del-mut-1 SARS-CoV-2 virus. Virus titration by plaque assay of lung and tracheal tissues collected on day 2 and 4 post-infection
The good news is that there already exist various furin and other protease inhibitors, and some of them (like camostat and its analogs) are already being clinically tested against CoV2.
By the way, it is possible that the new furin site could also be largely responsible for the pronounced age-dependent morbidity and mortality of CoV2:
Furin cuts proteins in strictly defined places, namely after an RxxR sequence (that is, Arg-X-X-Arg, where X can be any amino acid). Moreover, if arginine is also in the second or third place (that is, RRxR or RxRR), then the cleavage efficiency is significantly increased.
Therefore, the appearance of a new furin cleavage site was noticed immediately, as none of the closest or even distant relatives of Cov2 have such a site — those coronaviruses that do, share only 40% of their genome with Cov2:
Here is a great illustration from the source article of the quote above. Coronaviruses with a furin site are marked in pink, 3 different strains of Cov2 are shown at 10 o’clock:
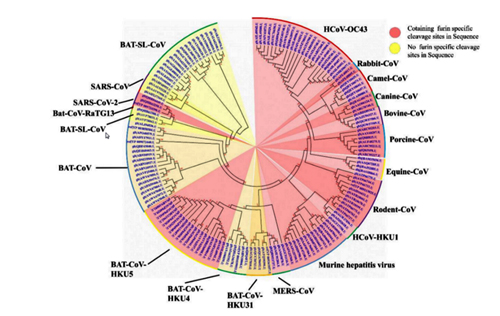
The closest relative with a furin site is the HKU5 strain, isolated by the Shi Zhengli team in 2014 in Guangzhou from bats of the genus Pipistrellus (added to GenBank in 2018). But it is a very distant relative — their spike proteins share only 36%.
So the virologists are puzzled. Where did this 12 nucleotide insert come from? Could it be lab-made? Well, virologists have studied furin sites in coronaviruses for decades, and have introduced many artificial ones in a lab. For example, an American team had inserted RRSRR into the spike protein of the first SARS-CoV back in 2006:

And the Japanese have inserted a similar site (RRKR) into the SARS-CoV protein in 2008, though a bit downstream than in CoV2:
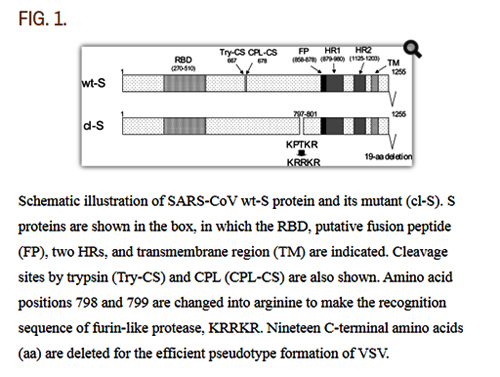
Schematic illustration of SARS-CoV wt-S protein and its mutant (cl-S). S proteins are shown in the box, in which the RBD, putative fusion peptide (FP), two HRs, and transmembrane region (TM) are indicated. Cleavage sites by trypsin (Try-CS) and CPL (CPL-CS) are also shown. Amino acid positions 798 and 799 are changed into arginine to make the recognition sequence of furin-like protease, KRRKR. Nineteen C-terminal amino acids (aa) are deleted for the efficient psuedotype formation of VSV.
In the same year 2008, their Dutch colleagues also studied these protease sites of SARS-CoV and compared them to the murine coronavirus MHV, which also has such a site (SRRAHR | SV), one that is quite similar to the site of CoV2 (SPRRAR | SV):
In 2009, another American group also worked on “improving” SARS-CoV and, continuing the American tradition of not penny-pinching on arginines, they inserted as many as 4 of them (RRSRR):
Beijing 2019
But the most recent work of this kind that I came across was an October 2019 paper from several Beijing labs, where the new furin site RRKR was inserted into not just some pseudovirus, but into an actual live chicken coronavirus, infectious bronchitis virus (IBV):
An interesting side note is that, as the authors point out, the addition of a furin site allows the mutant virus to infect nerve cells. Perhaps the CoV2 furin site is the reason why some patients with CoV2 exhibit neurological symptoms, including loss of smell:
To be clear, many coronaviruses have naturally occurring furin sites, and they are very diverse. Obviously, they can appear as a result of random mutations. This is what happened in the case of MERS, as was pointed out in 2015 by an international team of authors, including Shi Zhengli and Ralph Baric, two stars of synthetic coronavirusology. We will come back to them many times, but for now, a few words about that article. In it the authors have shown that just two mutations allowed MERS to jump from bats to humans, and one of these mutations created a furin site. Though it was not an insertion of new amino acids, but a mutation of an existing one (marked in red on the left below):
The authors did not just show this, but actually introduced these mutations back into the original bat strain: they created the same furin site and showed that it enables the bat strain to infect human cells:
By the way, how they did it might frighten those who aren’t familiar with modern biotechnology — because the authors inserted this coronavirus spike-like protein into inactivated HIV:
Perhaps this is what prompted Indian researchers to look for sequences similar to HIV in the CoV2 genome (but their preprint was quickly criticized for bad methodology and erroneous conclusions). In fact, experts use such pseudoviruses regularly, and in general, one should not be scared of retroviruses as a class — their subspecies lentiviruses have been used for gene therapy for many years.
Where Did RaTG13 Come From?
RaTG13 is a very unusual strain. Odd to see that Shi Zhengli’s group was silent about it for all these years. After all, it is very different from its SARS-like siblings, especially in the spike protein, which is precisely what determines which types of cells (and in which animals) this virus can infect. Here is a genome similarity graph of CoV2 compared to other bat coronaviruses (panel B):
The red curve represents RaTG13 while the blue curve is for the strains closest to RaTG13 (ZXC21 and ZC45). These strains were isolated from Chinese horseshoe bats (Rhinolophus sinicus) in Zhoushan in 2015 (ZXC21) and 2017 (ZC45). As can be seen from the above graph, even they differ in their S proteins from RaTG13. A direct sequence comparison illustrates this difference best:
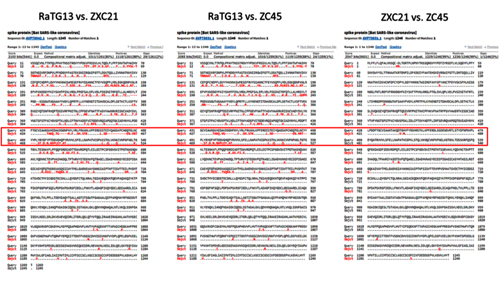
As we can see, the spike proteins of ZXC21 and ZC45 are not only 23–24 amino acid residues shorter than the RaTG13 protein, but they are shorter in the most important place — in the RBM (note the deletions in the red box marked with red dashes).
So where did RaTG13 come from? As I already mentioned, in 2020 Shi Zhengli reported that she isolated it in 2013 from Yunnan horseshoe bats (from Rhinolophus affinis, not the usual suspects R. sinicus). But until January 2020, this strain’s existence was not known, and here is how Shi Zhengli’s group described their discovery about RaTG13’s similarity to CoV2:
Not much detail: previously detected, and that is that. Moreover, the quote seems to imply that until 2020, they only sequenced a part of its genome, the RdRp gene (which is part of Orf1b that precedes the spike protein gene). Ok, but where exactly in Yunnan was it obtained? The paper doesn’t mention it, and neither does GenBank. However, the GISAID entry seems to have a bit more info: collected in Pu’er City from a male bat’s fecal swab:

This rang a bell, as in my wanderings around Pubmed, I had already encountered an expedition to Pu’er in the summer of 2013:
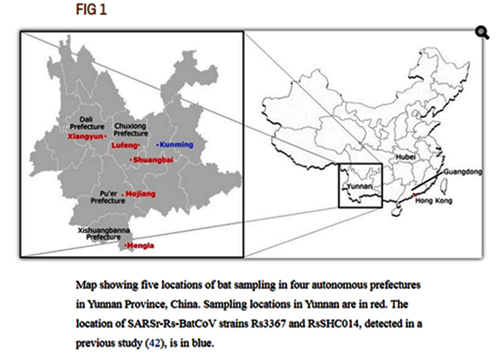
Map showing five locations of bat sampling in four autonomous prefectures in Yunnan Province, China. Sampling locations in Yunnan are in red. The location of SARSr-Rs-BatCoV strains Rs3367 and RsSHC014, detected in a previous study (42), is in blue.
Researchers did not report anything particularly interesting for us from that expedition, but maybe it was then that Shi Zhengli or someone from her group obtained the RaTG13 sample? Which they sequenced only partially, and for some reason decided not to publish, although it was very different from everything known before.
By the way, Shi Zhengli could well have personally participated in that expedition, as she expressed great fondness when describing them — for example, in her TED-like talk in 2018, where she showed personal photos from such expeditions:
CREATOR OF NEW CORONAVIRUS? WUHAN INSTITUTE OF VIROLOGY
Moreover, it was a series of exactly such expeditions that brought Shi Zhengli worldwide fame and a “Batwoman” moniker: in a 2013 Nature paper, her group triumphantly announced that in Yunnan caves they had discovered carrier bats of the RsSHC014 and Rs3367 strains that coincided with the first SARS-CoV by 85% and 96%, respectively.
It is quite a coincidence that around the same time in Yunnan, Shi Zhengli’s group also discovered RaTG13, the closest strain to CoV2, and the two also share 96% of their genomes.
UPD: Is RaTG13 the same as RaBtCoV/4991?
[UPDATED] After I had published this post, I was pointed to this preprint that alleges that RaTG13 is, in fact, RaBtCoV/4991 (KP876546), which Shi Zhengli had previously reported discovering in an abandoned mineshaft in Yunnan in 2013. There indeed are several reasons to think so. First and foremost, the only published sequence for RaBtCoV/4991 is 100% identical to that of RaTG13 at the nucleotide level, albeit being just a 370-bp stretch of the RdRp gene:
Second, the collection details of the two strains are nearly identical: both were collected in July 2013 from a fecal swab of R. affinis bats:

RaBtCoV/4991 was collected in a mineshaft located in the Mojiang county, which is under the jurisdiction of Pu’er City:
And Pu’er City is listed as the collection location of RaTG13 at the GISAID database, which could well be an approximation for the Mojiang mineshaft.
It is odd that in her 2020 paper on RaTG13 Shi Zhengli fails to mention RaBtCoV/4991 or cite her 2016 paper about its discovery, for which she is listed as the one who “designed and coordinated the study”. It is not like RaBtCoV/4991 was forgotten by her group, as it is mentioned in their 2019 paper, where it is included in a phylogenetic tree of other coronaviruses:
Sampling map (A) and phylogenetic analysis of CoVs detected in Rhinolophus bats (B). A total of 19 provinces (indicated in gray) in China were involved. 1. Beijing (BJ), 2. Chongquing (CA); 3. Fujian (FJ); 4. Gansu (GS); 5. Guangdong (GD); 6. Guangxi (BX); 7. Guizhou (GZ); 8. Hainan (HaN); 9. Hebei (HeB); 10. Henan (HeN); 11. Hubei (HuB); 12. Hunan (HuN); 13. Jiangsu (JS); 14. Shandong (SD); 15. Shanxi (SX); 16. Sichuan (SC); 17. Tibet (T); 18. Yunnan (YN); and 19. Zhejiang (ZJ). The partial sequences of RdRp gene (327-bp) of CoVs detected in Rhinolophus bats were aligned with those of published represenative CoV strains. The tree was constructed by the maximum-likelihood method with bootstrap values determined with 1000 replicates. The scale bar indicates the estimated number of substitutions per 10 nucleotides. Filled triangles indicate the CoVs published previously by our lab (KU343197, KP876536, KP876544, MF094687, KP876546, KY417143, FJ588686) [15.18.40.41], filled diamonds indicate CoVs detected in this study. Putative novel alphaCoVs are labeled in green. BtCoV/Rh/YN2012 detected in Guangdong and Yunnan province in this study are in bold. FIPV, Feline infectious peritonitis virus; PEDV, procine epidemic diarrhea virus; MHV, mouse hepatitis virus. Other abbreviations are defined as those in the text. Numbers in parentheses indicate numbers of sequences sharing >97% identity.
I doubt that RaBtCoV/4991’s place in that tree was determined based solely on a 370-bp fragment, so I would think that by early 2019, Shi Zhengli’s group would have sequenced its full genome.
Intriguingly, both pangolin-2017 and pangolin-2019 genomes are also very close in this stretch of the RdRp gene, and CoV2 and pangolin-2019 share a few common mutations not found in RaTG13:

But let’s put this topic aside for now and get back to the story of Shi Zhengli’s famous 2013 Nature paper.
Lab-Made? SARS-CoV-2 Genealogy Through the Lens of Gain-of-Function Research
by Yuri Deigin
Apr 22, 2020
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT
YOU ARE REQUIRED TO READ THE COPYRIGHT NOTICE AT THIS LINK BEFORE YOU READ THE FOLLOWING WORK, THAT IS AVAILABLE SOLELY FOR PRIVATE STUDY, SCHOLARSHIP OR RESEARCH PURSUANT TO 17 U.S.C. SECTION 107 AND 108. IN THE EVENT THAT THE LIBRARY DETERMINES THAT UNLAWFUL COPYING OF THIS WORK HAS OCCURRED, THE LIBRARY HAS THE RIGHT TO BLOCK THE I.P. ADDRESS AT WHICH THE UNLAWFUL COPYING APPEARED TO HAVE OCCURRED. THANK YOU FOR RESPECTING THE RIGHTS OF COPYRIGHT OWNERS.

Staff celebrating the physical completion of the laboratory in 2015, Wuhan, China (Source)
If you hear anyone claim “we know the virus didn’t come from a lab”, don’t buy it — it may well have. Labs around the globe have been creating synthetic viruses like CoV2 for years. And no, its genome would not necessarily contain hallmarks of human manipulation: modern genetic engineering tools permit cutting and pasting genomic fragments without leaving a trace. It can be done quickly, too: it took a Swiss team less than a month to create a synthetic clone of CoV2.
How I Learned to Start Worrying
Oh, come on. Lab-made? Nonsense! Back in January, that was my knee-jerk reaction when ideas that Covid-19 is caused by a laboratory leak had just surfaced. Bioweapon? Well, that is just Flat Earth crazies territory. Thus, whenever I kept hearing anything about non-natural origins of SARS-CoV-2, I brushed it aside under similar sentiments. So what if there is a virology institute in Wuhan? Who knows how many of those are sprinkled throughout China.
At some point, it became necessary to brush such theories aside in a substantiated manner, as their proponents began to back up their theses about the possible artificial nature of the virus with arguments from molecular biology, and when engaging them in debate, I wanted to smash their conspiracy theories with cold, hard scientific facts. Just like that Nature paper (or so I thought).
So it was then, in pursuit of arguments against the virus’s lab-madeness, that I got infected by the virus of doubt. What was the source of my doubts? The fact that the deeper you dive into the research activities of coronavirologists over the past 15–20 years, the more you realize that creating chimeras like CoV2 was commonplace in their labs.
A chimera virus is defined by the Center for Veterinary Biologics (part of the U.S. Department of Agriculture's Animal and Plant Health Inspection Service) as a "new hybrid microorganism created by joining nucleic acid fragments from two or more different microorganisms in which each of at least two of the fragments contain essential genes necessary for replication." The term chimera already referred to an individual organism whose body contained cell populations from different zygotes or an organism that developed from portions of different embryos. In mythology, a chimera is a creature such as a hippogriff or a gryphon formed from parts of different animals, thus the name for these viruses. Chimeric flaviviruses have been created in an attempt to make novel live attenuated vaccines.
-- Chimera (virus), by Wikipedia
And CoV2 is an obvious chimera (though not necessarily a lab-made one), which is based on the ancestral bat strain RaTG13, in which the receptor binding motif (RBM) in its spike protein is replaced by the RBM from a pangolin strain, and in addition, a small but very special stretch of 4 amino acids is inserted, which creates a furin cleavage site that, as virologists have previously established, significantly expands the “repertoire” of the virus in terms of whose cells it can penetrate. Most likely, it was thanks to this new furin site that the new mutant managed to jump species from its original host to humans.
Indeed, virologists, including the leader of coronavirus research at the Wuhan Institute of Virology, Shi Zhengli, have done many similar things in the past — both replacing the RBM in one type of virus by an RBM from another, or adding a new furin site that can provide a species-specific coronavirus with an ability to start using the same receptor (e.g. ACE2) in other species. In fact, Shi Zhengli’s group was creating chimeric constructs as far back as 2007 and as recently as 2017, when they created a whole of 8 new chimeric coronaviruses with various RBMs. In 2019 such work was in full swing, as WIV was part of a $3.7 million NIH grant titled Understanding the Risk of Bat Coronavirus Emergence. Under its auspices, Shi Zhengli co-authored a 2019 paper that called for continued research into synthetic viruses and testing them in vitro and in vivo:
Currently, no clinical treatments or prevention strategies are available for any human coronavirus. Given the conserved RBDs of SARS-CoV and bat SARSr-CoVs, some anti-SARS-CoV strategies in development, such as anti-RBD antibodies or RBD-based vaccines, should be tested against bat SARSr-CoVs. Recent studies demonstrated that anti-SARS-CoV strategies worked against only WIV1 and not SHC014. In addition, little information is available on HKU3-related strains that have much wider geographical distribution and bear truncations in their RBD. Similarly, anti-S antibodies against MERS-CoV could not protect from infection with a pseudovirus bearing the bat MERSr-CoV S. Furthermore, little is known about the replication and pathogenesis of these bat viruses. Thus, future work should be focused on the biological properties of these viruses using virus isolation, reverse genetics and in vitro and in vivo infection assays. The resulting data would help the prevention and control of emerging SARS-like or MERS-like diseases in the future.
If the above quote might seem vague as to what exactly “using reverse genetics” might mean, the NIH grant itself spells it out:
Aim 3. In vitro and in vivo characterization of SARSr-CoV spillover risk, coupled with spatial and phylogenetic analyses to identify the regions and viruses of public health concern. We will use S protein sequence data, infectious clone technology, in vitro and in vivo infection experiments and analysis of receptor binding to test the hypothesis that % divergence thresholds in S protein sequences predict spillover potential.
“Infectious clone technology” stands for creating live synthetic viral clones. Considering the heights of user friendliness and automation that genetic engineering tools have attained, creating a synthetic CoV2 via the above methodology would be in reach of even a grad student.
But before delving into CoV2 origins, let’s first take a quick dive into its biology.
Biology
Ok, let’s start from the basics. What’s a furin site, an RBM, or a spike protein? Bear with me: once you wade through the jungle of terminology, conceptually, everything is pretty straightforward. For example, spike proteins are those red things sticking out of a virus particle — the very reason for which these viruses got “crowned”:

It is with the help of these proteins that the virion clings to the receptor of the victim cell (ACE2 in our case) to then penetrate inside. So it is a vitally important part of the virus, as without getting into a cell viruses cannot replicate. The spike protein also determines which animals the virus can or cannot infect, as ACE2 receptors (or other targets for other viruses) in different species can differ in structure. At the same time, out of the entire 30 kilobase genome (quite huge by viral standards), the gene of this protein makes up only 12–13%. So the spike protein is only about 1300 amino acids long. Below is how the spike (S) protein is structured in CoV2 and close relatives:

As can be seen from the figure above, the S protein consists of two subunits: S1 and S2. It is S1 that interacts with the ACE2 receptor, and the place where S1 does so is called Receptor Binding Domain (RBD), while the area of direct contact, the holy of holies, is called Receptor Binding Motif (RBM). Here is a beautiful illustration from an equally beautiful work:

Overall structure of 2019-nCoV RBD bound with ACE2.
(a) Overall topology of 2019-nCoV spike monomer. NTD, N-terminal domain. RBD, receptor-binding domain. RBM, receptor-binding motif. SD1, subdomain 1. SD2, subdomain 2. FP, fusion peptide. HR1, heptad repeat 1. HR2, heptad repeat 2. TM, transmembrane region. IC, intracellular domain.
(b) Sequence and secondary structures of 2019-nCoV RBD. The RBM is colored red.
© Overall structure of 2019-nCoV RBD bound with ACE2. ACE2 is colored green. 2019-nCoV RBD core is colored cyan and RBM is colored red. Disulfide bonds in the 2019-nCoV RBD are shown as stick and indicated by yellow arrows. The N-terminal helix of ACE2 responsible for binding is labeled.
When the CoV2 genome was just sequenced and made publicly available on January 10, 2020, it was a riddle, as no closely related strains were known. But quite quickly, on January 23, Shi Zhengli released a paper indicating that CoV2 is 96% identical to RaTG13, a strain which her laboratory had previously isolated from Yunnan bats in 2013. However, outside of her lab, no one knew about that strain until January 2020.
It was immediately clear that RaTG13 is special. Take a look at the figure below:

This is a genome similarity graph between CoV2 and other known strains. The higher the curve, the higher the percentage of matching nucleotides. As you can see, in the spike protein (S) gene region (between nucleotides 22k and 25k), only RaTG13 is more or less close to CoV2, while all other strains take a deep dive around this spot — both strains from other bats and the first SARS-CoV (red curve). This in itself is far from suspicious — who knows how many unknown SARS-like strains lurk in the bat caves of Yunnan? Ok, maybe it is not very clear how exactly the virus could get from there to Wuhan, but hey, with those wet markets you never know.
Pangolins
Next, pangolins appeared on the scene: in February, another group of Chinese scientists discovered a peculiar strain of pangolin coronavirus in their possession, which, while generally being only 90% similar to CoV2, in the RBM region was almost identical to it, with only a single amino acid difference (see the upper two sequences, dots indicate a match with the top sequence):

Surprisingly, in the first quarter of the S protein, the pangolin strain is highly dissimilar from CoV2, but after the RBM all three strains (CoV2, Pangolin, RaTG13) exhibit a shared high degree of similarity. Most strikingly, RaTG13’s RBM itself is quite different than that of CoV2, which can be seen from the steep dive of the green RaTG13 graph compared to the red CoV2 graph in the RBM region (pink strip) in the following graph:

This observation is confirmed by the phylogenetic analysis of the three areas highlighted in the graph above — in the RBM, the pangolin strain is closer to CoV2 than is RaTG13, but it is RaTG13 that is closer to CoV2 to the left and right of RBM. So there is obvious recombination, as the authors (and other papers) conclude.
Genetic recombination (also known as genetic reshuffling) is the exchange of genetic material between different organisms which leads to production of offspring with combinations of traits that differ from those found in either parent.
-- Genetic recombination, by Wikipedia
How did the researchers obtain those pangolins? This is how:

They were confiscated from smugglers by Chinese customs and transferred to an animal rehab center in Guangdong, where they died while exhibiting severe coronavirus symptoms. This, of course, must have gotten the attention of local virologists, who took several samples:
Pangolins used in the study were confiscated by Customs and Department of Forestry of Guangdong Province in March-December 2019. They include four Chinese pangolins (Manis pentadactyla) and 25 Malayan pangolins (Manis javanica). These animals were sent to the wildlife rescue center, and were mostly inactive and sobbing, and eventually died in custody despite exhausting rescue efforts. Tissue samples were taken from the lung, lymph nodes, liver, spleen, muscle, kidney, and other tissues from pangolins that had just died for histopathological and virological examinations.
Those pangolins attracted the attention of other virologists too. For example, a team in Hong Kong also received samples of confiscated pangolins and in February 2020 they also released a paper that noted clear signs of recombination in the CoV2 spike protein:
We received frozen tissue (lungs, intestine, blood) samples that were collected from 18 Malayan pangolins (Manis javanica) during August 2017-January 2018. These pangolins were obtained during the anti-smuggling operations by Guangxi Customs. Strikingly, high-throughput sequencing of their RNA revealed the presence of coronaviruses in six (two lung, two intestine, one lung-intestine mix, one blood) of 43 samples. With the sequence read data, and by filling gaps with amplicon sequencing, we were able to obtain six full or nearly full genome sequences — denoted GX/P1E, GX/P2V, GX/P3B, GX/P4L, GX/P5E and GX/P5L — that fall into the 2019-CoV2 lineage (within the genus Betacoronavirus) in a phylogenetic analysis (Figure 1a).
…
More notable, however, was the observation of putative recombination signals between the pangolins coronaviruses, bat coronaviruses RaTG13, and human 2019-CoV2 (Figure 1c, d). In particular, 2019-CoV2 exhibits very high sequence similarity to the Guangdong pangolin coronaviruses in the receptor-binding domain (RBD; 97.4% amino acid similarity; indicated by red arrow in Figure 1c and Figure 2a), even though it is most closely related to bat coronavirus RaTG13 in the remainder of the viral genome. Bat CoV RaTG and the human 2019-CoV2 have only 89.2% amino acid similarity in RBD. Indeed, the Guangdong pangolin coronaviruses and 2019-CoV2 possess identical amino acids at the five critical residues of the RBD, whereas RaTG13 only shares one amino acid with 2019-CoV2 (residue 442, human SARS-CoV numbering).
By the way, the authors of this article also highlighted the high phylogenetic mosaicity of the CoV2 spike protein:
Interestingly, a phylogenetic analysis of synonymous sites alone in the RBD revealed that the phylogenetic position of the Guangdong pangolin is consistent with that in the remainder of the viral genome, rather than being the closest relative of 2019-CoV2 (Figure 2b). Hence, it is possible that the amino acid similarity between the RBD of the Guangdong pangolin coronaviruses and 2019-CoV2 is due to selectively-mediated convergent evolution rather than recombination, although it is difficult to choose between these scenarios on current data.
Translated from science-speak, what this means is that if we analyze the entire RBD of the three strains, ignoring the obvious differences (i.e. non-synonymous substitutions) among them, which are mainly found in the RBM (which, recall, is identical between CoV2 and Pangolin), and construct a phylogenetic tree for synonymous substitutions, CoV2 is still closer to RaTG13 than to the pangolin strain. Which is rather strange in light of the fact that the pangolin strain and CoV2 have identical RBMs (which are segments inside RBD).
The authors go on to put forth a conjecture that this may be the result of convergent evolution, in other words, that CoV2 and the pangolin strain came to possess identical RBMs each in their own way, rather than through recombination between common ancestors. Because it would have required a rather unique recombination event — as if someone cut out a precise RBM segment from a pangolin strain and used it to replace the RBM in RaTG13. Talk about Intelligent Design!
Royal Genealogy
In order to better understand CoV2 origins, let’s take a look at spike protein sequences of our Unholy Trinity: CoV2, RaTG13 and MP789 (pangolin-2019). Let’s compare the pairwise differences between them (identical amino acids are marked with dots, red letters denote differences, and dashes indicate deleted/inserted amino acids):

The comparisons illustrate what previously quoted papers have noted: that in the first quarter of the sequence, the pangolin strain is far from CoV2 and RaTG1, and if it weren’t for the RBM region (red rectangle), RaTG13 would have been very close to CoV2. But, as I already said, the RBM in CoV2 is closest to that of the pangolin strain.
What about other pangolin strains? So far we’ve only analyzed the MP789 strain isolated from pangolins confiscated by customs in 2019. But there was another batch of pangolins confiscated in 2017, and they also had a similar coronavirus strain isolated. Let’s compare it to RaTG13 and MP789:

In the first quarter of the S protein, the 2017 pangolin strains are closer to RaTG13 (and CoV2) than their 2019 pangolin counterpart (MP789). At the same time, all three have a clear recent common ancestor in the areas marked by green rectangles, and in these areas RaTG13 and pangolin-2019 (MP789) are closer to each other than to pangolin-2017, since they have several common mutations (marked by red and blue ellipses), which are absent from pangolin-2017. But the RBM for all three is different, and different in approximately the same proportion, and in similar places.
Maybe after ancestors of RaTG13 and MP789 diverged, the MP789 ancestor had the first quarter of its protein replaced (which did not occur in RaTG13 or pangolin-2017), and the rest of the protein remained common for all three strains. Later the paths of the RaTG13 and MP789 gene pools crossed again and produced CoV2. It is also possible that the ancestor of RaTG13 arose as a result of recombination of ancestral pangolin strains.
It is also interesting to see a rather unique identical mutation (QTQTNS) in RaTG13 and pangolin-2019 right in front of the spot where CoV2 has a new furin cleavage site. That furin site, as I mentioned, arose via an insertion of 4 new amino acids (PRRA). If we look at the nucleotide sequence around this insertion, we can see that RaTG13 and CoV2 are closer to each other in that area than to pangolin-2019, since they possess several common mutations (highlighted in blue):

By the way, Orf1ab is also a phylogenetic mess in CoV2: 1a is closer to RaTG13, but 1b is closer to pangolin-2019:

(Image Source)
Does this mean that the ancestor of CoV2 crossed with the common ancestor of pangolin-19 at least twice? First, when it (along with a common ancestor of RaTG13) inherited Orf1ab and the second half of the spike protein with the QTQTNS mutation, and second time when it acquired 1b and RBM, which differ from RaTG13. All of this is certainly possible in nature — after all, these viruses mutate and recombine constantly. Another question is where exactly bat and pangolin viruses are most likely to encounter one another for such orgies — in mountain caves, “wet markets”, shelters for confiscated animals, or even in laboratories. But let’s put those questions aside for now. First, let's discuss what is arguably the most eye-catching aspect of the new virus — a 4-amino acid insertion that turned it into a natural-born killer.
A Killer Intro
It is impossible to ignore the introduction of a PRRA insert between S1 and S2: it sticks out like a splinter. This insert creates the furin cleavage site, which I mentioned at the very beginning. Let me explain what a furin site is. Remember the structure of our spike protein? Here is a detailed diagram:

The protein consists of two parts, S1 and S2, of which S1 is responsible for primary contact with the receptor (recall Receptor Binding Domain / Motif), and S2 is responsible for fusion with the cell membrane and penetration into the cell. The fusion process is started by the fusion peptide marked in yellow, but in order for it to engage in its dirty deed, someone must cut the S protein at one of the sites marked by diamonds in the diagram above. The virus does not have its own such “cutters”, so it relies on various proteases of its victims. There are several types of such proteases, as can be deduced from the abundance of colors of those diamonds. But not all proteases are equal, and not all types of cells have proteases needed by the virus. Furin is one of the most effective, and it is found not only on the surface of cells, but also inside. Most clearly, the danger of the new furin site is demonstrated by the difference between CoV2 and its grandpa, SARS-CoV:

As can be seen from the diagram, in the case of CoV2, thanks to the furin site, it is not two, but three classes of proteases (three colored PacMans) that can cut its S protein outside the cell. But perhaps the most important difference is that furin is also present inside the cell, so it can cut the S protein immediately after virion assembly, thereby providing new virions with the ability to merge with new cells right off the bat (no pun intended).
The importance of the new furin site in CoV2’s virulence was recently demonstrated by a study in hamsters where the disappearance of the furin site (due to a mutation) greatly decreased mutant CoV2’s pathogenicity and replication ability:
Infection of hamsters shows that one of the variants (Del-mut-1) which carries deletion of 10 amino acids (30 bp) does not cause the body weight loss or more severe pathological changes in the lungs that is associated with wild type virus infection.

Virus replication in the lung tissues of hamsters infected with either WT or Del-mut-1 SARS-CoV-2 virus. Virus titration by plaque assay of lung and tracheal tissues collected on day 2 and 4 post-infection
The good news is that there already exist various furin and other protease inhibitors, and some of them (like camostat and its analogs) are already being clinically tested against CoV2.
By the way, it is possible that the new furin site could also be largely responsible for the pronounced age-dependent morbidity and mortality of CoV2:
Patients with hypertension, diabetes, coronary heart disease, cerebrovascular illness, chronic obstructive pulmonary disease, and kidney dysfunction have worse clinical outcomes when infected with SARS-CoV-2, for unknown reasons. The purpose of this review is to summarize the evidence for the existence of elevated plasmin(ogen) in COVID-19 patients with these comorbid conditions. Plasmin, and other proteases, may cleave a newly inserted furin site in the S protein of SARS-CoV-2, extracellularly, which increases its infectivity and virulence.
Plasmin is an important enzyme (EC 3.4.21.7) present in blood that degrades many blood plasma proteins, including fibrin clots...
Plasmin is a serine protease that acts to dissolve fibrin blood clots. Apart from fibrinolysis, plasmin proteolyses proteins in various other systems: It activates collagenases, some mediators of the complement system, and weakens the wall of the Graafian follicle, leading to ovulation. It cleaves fibrin, fibronectin, thrombospondin, laminin, and von Willebrand factor. Plasmin, like trypsin, belongs to the family of serine proteases.
-- Plasmin, by Wikipedia
Furin cuts proteins in strictly defined places, namely after an RxxR sequence (that is, Arg-X-X-Arg, where X can be any amino acid). Moreover, if arginine is also in the second or third place (that is, RRxR or RxRR), then the cleavage efficiency is significantly increased.
Therefore, the appearance of a new furin cleavage site was noticed immediately, as none of the closest or even distant relatives of Cov2 have such a site — those coronaviruses that do, share only 40% of their genome with Cov2:
It was found that all Spike with a SARS-CoV-2 Spike sequence homology greater than 40% did not have a furin cleavage site (Figure 1, Table 1), including Bat-CoV RaTG13 and SARS-CoV (with sequence identity as 97.4% and 78.6%, respectively). The furin cleavage site “RRAR” in SARS-CoV-2 is unique in its family, rendering by its unique insert of “PRRA”. The furin cleavage site of SARS-CoV-2 is unlikely to have evolved from MERS, HCoV-HKU1, and so on. From the currently available sequences in databases, it is difficult for us to find the source. Perhaps there are still many evolutionary intermediate sequences waiting to be discovered.
Here is a great illustration from the source article of the quote above. Coronaviruses with a furin site are marked in pink, 3 different strains of Cov2 are shown at 10 o’clock:

The closest relative with a furin site is the HKU5 strain, isolated by the Shi Zhengli team in 2014 in Guangzhou from bats of the genus Pipistrellus (added to GenBank in 2018). But it is a very distant relative — their spike proteins share only 36%.
So the virologists are puzzled. Where did this 12 nucleotide insert come from? Could it be lab-made? Well, virologists have studied furin sites in coronaviruses for decades, and have introduced many artificial ones in a lab. For example, an American team had inserted RRSRR into the spike protein of the first SARS-CoV back in 2006:
To investigate whether proteolytic cleavage at the basic amino acid residues, were it to occur, might facilitate cell–cell fusion activity, we mutated the wild-type SARS-CoV glycoprotein to construct a prototypic furin recognition site (RRSRR) at either position.

And the Japanese have inserted a similar site (RRKR) into the SARS-CoV protein in 2008, though a bit downstream than in CoV2:

Schematic illustration of SARS-CoV wt-S protein and its mutant (cl-S). S proteins are shown in the box, in which the RBD, putative fusion peptide (FP), two HRs, and transmembrane region (TM) are indicated. Cleavage sites by trypsin (Try-CS) and CPL (CPL-CS) are also shown. Amino acid positions 798 and 799 are changed into arginine to make the recognition sequence of furin-like protease, KRRKR. Nineteen C-terminal amino acids (aa) are deleted for the efficient psuedotype formation of VSV.
In the same year 2008, their Dutch colleagues also studied these protease sites of SARS-CoV and compared them to the murine coronavirus MHV, which also has such a site (SRRAHR | SV), one that is quite similar to the site of CoV2 (SPRRAR | SV):

In 2009, another American group also worked on “improving” SARS-CoV and, continuing the American tradition of not penny-pinching on arginines, they inserted as many as 4 of them (RRSRR):
To examine the potential use of the SARS-CoV S1–S2 and S2′ positions as sites for proteolytic cleavage, we first introduced furin cleavage recognition sites at these locations by making the following mutations 664-SLLRSTSQSI — SLLRRSRRSI-671 (S1–S2) and 792-LKPTKRSF — LKRTKRSF-799 (S2′).
Beijing 2019
But the most recent work of this kind that I came across was an October 2019 paper from several Beijing labs, where the new furin site RRKR was inserted into not just some pseudovirus, but into an actual live chicken coronavirus, infectious bronchitis virus (IBV):

An interesting side note is that, as the authors point out, the addition of a furin site allows the mutant virus to infect nerve cells. Perhaps the CoV2 furin site is the reason why some patients with CoV2 exhibit neurological symptoms, including loss of smell:
Mutation of the S2' site of QX genotype (QX-type) spike protein (S) in a recombinant virus background results in higher pathogenicity, pronounced neural symptoms and neurotropism when compared with conditions in wild-type IBV (WT-IBV) infected chickens. In this study, we present evidence suggesting that recombinant IBV with a mutant S2' site (furin-S2' site) leads to higher mortality. Infection with mutant IBV induces severe encephalitis and breaks the blood–brain barrier.
…
In summary, our results demonstrate that the furin cleavage site upstream of the FP in S protein is an important site for CoV, modulating entry, cell–virus fusion, adaptation to its host cell, cell tropism and pathogenicity, but not antigenicity.
Encephalitis is inflammation of the brain. There are several causes, but the most common is a viral infection.
Encephalitis often causes only mild flu-like signs and symptoms — such as a fever or headache — or no symptoms at all. Sometimes the flu-like symptoms are more severe. Encephalitis can also cause confused thinking, seizures, or problems with movement or with senses such as sight or hearing.
In some cases, encephalitis can be life-threatening. Timely diagnosis and treatment are important because it's difficult to predict how encephalitis will affect each individual.
Encephalitis, by Mayo Clinic
To be clear, many coronaviruses have naturally occurring furin sites, and they are very diverse. Obviously, they can appear as a result of random mutations. This is what happened in the case of MERS, as was pointed out in 2015 by an international team of authors, including Shi Zhengli and Ralph Baric, two stars of synthetic coronavirusology. We will come back to them many times, but for now, a few words about that article. In it the authors have shown that just two mutations allowed MERS to jump from bats to humans, and one of these mutations created a furin site. Though it was not an insertion of new amino acids, but a mutation of an existing one (marked in red on the left below):

The authors did not just show this, but actually introduced these mutations back into the original bat strain: they created the same furin site and showed that it enables the bat strain to infect human cells:
To evaluate the potential genetic changes required for HKU4 to infect human cells, we reengineered HKU4 spike, aiming to build its capacity to mediate viral entry into human cells. To this end, we introduced two single mutations, S746R and N762A, into HKU4 spike. The S746R mutation was expected to restore the hPPC motif in HKU4 spike, whereas the N762A mutation likely disrupted the potential N-linked glycosylation site in the hECP motif in HKU4 spike.
…
We examined the capability of the mutant HKU4 spike to mediate viral entry into three types of human cells (Fig. 3A for HEK293T cells; data not shown for Huh-7 and MRC-5 cells), using a pseudovirus entry assay as previously described (14). In the absence of exogenous protease trypsin, HKU4 pseudoviruses bearing either the reengineered hPPC motif or the reengineered hECP motif were able to enter human cells, whereas HKU4 pseudoviruses bearing both of the reengineered human protease motifs entered human cells as efficiently as when activated by exogenous trypsin (Fig. 3A). In contrast, wild-type HKU4 pseudoviruses failed to enter human cells. Therefore, the reengineered hPPC and hECP motifs enabled HKU4 spike to be activated by human endogenous proteases and thereby allowed HKU4 pseudoviruses to bypass the need for exogenous proteases to enter human cells. These results reveal that HKU4 spike needs only two single mutations at the S1/S2 boundary to gain the full capacity to mediate viral entry into human cells.
By the way, how they did it might frighten those who aren’t familiar with modern biotechnology — because the authors inserted this coronavirus spike-like protein into inactivated HIV:
Briefly, MERS-CoV-spike-pseudotyped retroviruses expressing a luciferase reporter gene were prepared by cotransfecting HEK293T cells with a plasmid carrying Env-defective, luciferase-expressing HIV-1 genome (pNL4–3.luc.R-E-) and a plasmid encoding MERS-CoV spike protein.
Perhaps this is what prompted Indian researchers to look for sequences similar to HIV in the CoV2 genome (but their preprint was quickly criticized for bad methodology and erroneous conclusions). In fact, experts use such pseudoviruses regularly, and in general, one should not be scared of retroviruses as a class — their subspecies lentiviruses have been used for gene therapy for many years.
Where Did RaTG13 Come From?
RaTG13 is a very unusual strain. Odd to see that Shi Zhengli’s group was silent about it for all these years. After all, it is very different from its SARS-like siblings, especially in the spike protein, which is precisely what determines which types of cells (and in which animals) this virus can infect. Here is a genome similarity graph of CoV2 compared to other bat coronaviruses (panel B):

The red curve represents RaTG13 while the blue curve is for the strains closest to RaTG13 (ZXC21 and ZC45). These strains were isolated from Chinese horseshoe bats (Rhinolophus sinicus) in Zhoushan in 2015 (ZXC21) and 2017 (ZC45). As can be seen from the above graph, even they differ in their S proteins from RaTG13. A direct sequence comparison illustrates this difference best:

As we can see, the spike proteins of ZXC21 and ZC45 are not only 23–24 amino acid residues shorter than the RaTG13 protein, but they are shorter in the most important place — in the RBM (note the deletions in the red box marked with red dashes).
So where did RaTG13 come from? As I already mentioned, in 2020 Shi Zhengli reported that she isolated it in 2013 from Yunnan horseshoe bats (from Rhinolophus affinis, not the usual suspects R. sinicus). But until January 2020, this strain’s existence was not known, and here is how Shi Zhengli’s group described their discovery about RaTG13’s similarity to CoV2:
We then found that a short region of RNA-dependent RNA polymerase (RdRp) from a bat coronavirus (BatCoV RaTG13) — which was previously detected in Rhinolophus affinis from Yunnan province — showed high sequence identity to 2019-CoV2. We carried out full-length sequencing on this RNA sample (GISAID accession number EPI_ISL_402131). Simplot analysis showed that 2019-CoV2 was highly similar throughout the genome to RaTG13 (Fig. 1c), with an overall genome sequence identity of 96.2%.
Not much detail: previously detected, and that is that. Moreover, the quote seems to imply that until 2020, they only sequenced a part of its genome, the RdRp gene (which is part of Orf1b that precedes the spike protein gene). Ok, but where exactly in Yunnan was it obtained? The paper doesn’t mention it, and neither does GenBank. However, the GISAID entry seems to have a bit more info: collected in Pu’er City from a male bat’s fecal swab:

This rang a bell, as in my wanderings around Pubmed, I had already encountered an expedition to Pu’er in the summer of 2013:
Bats were captured from various locations in five counties of four prefectures of Yunnan Province, China, from May to July 2013.

Map showing five locations of bat sampling in four autonomous prefectures in Yunnan Province, China. Sampling locations in Yunnan are in red. The location of SARSr-Rs-BatCoV strains Rs3367 and RsSHC014, detected in a previous study (42), is in blue.
Researchers did not report anything particularly interesting for us from that expedition, but maybe it was then that Shi Zhengli or someone from her group obtained the RaTG13 sample? Which they sequenced only partially, and for some reason decided not to publish, although it was very different from everything known before.
By the way, Shi Zhengli could well have personally participated in that expedition, as she expressed great fondness when describing them — for example, in her TED-like talk in 2018, where she showed personal photos from such expeditions:
CREATOR OF NEW CORONAVIRUS? WUHAN INSTITUTE OF VIROLOGY
Moreover, it was a series of exactly such expeditions that brought Shi Zhengli worldwide fame and a “Batwoman” moniker: in a 2013 Nature paper, her group triumphantly announced that in Yunnan caves they had discovered carrier bats of the RsSHC014 and Rs3367 strains that coincided with the first SARS-CoV by 85% and 96%, respectively.
It is quite a coincidence that around the same time in Yunnan, Shi Zhengli’s group also discovered RaTG13, the closest strain to CoV2, and the two also share 96% of their genomes.
UPD: Is RaTG13 the same as RaBtCoV/4991?
[UPDATED] After I had published this post, I was pointed to this preprint that alleges that RaTG13 is, in fact, RaBtCoV/4991 (KP876546), which Shi Zhengli had previously reported discovering in an abandoned mineshaft in Yunnan in 2013. There indeed are several reasons to think so. First and foremost, the only published sequence for RaBtCoV/4991 is 100% identical to that of RaTG13 at the nucleotide level, albeit being just a 370-bp stretch of the RdRp gene:
BtCoV/4991 was first described in 2016. It is a 370 nucleotide virus fragment collected from the Mojiang mine in 2013 by the lab of Zeng-li Shi at the WIV [Wuhan Institute of Virology] (Ge et al., 2016). BtCoV/4991 is 100% identical in sequence to one segment of RaTG13. RaTG13 is a complete viral genome sequence (almost 30,000 nucleotides) that was only published in 2020, after the pandemic began (P. Zhou et al., 2020).
Despite the confusion created by their different names, in a letter obtained by us Zheng-li Shi confirmed to a virology database that BtCoV/4991 and RaTG13 are both from the same bat faecal sample and the same mine. They are thus sequences from the same virus...
Why did the Shi lab not acknowledge the miners’ deaths in any paper describing samples taken from the mine (Ge et al., 2016 and P. Zhou et al., 2020)? Why in the title of the Ge at al. 2016 paper did the Shi lab call it an “abandoned” mine? When they published the sequence of RaTG13 in Feb. 2020, why did the Shi lab provide a new name (RaTG13) for BtCoV/4991 when they had by then cited BtCoV/4991 twice in publications and once in a genome sequence database and when their sequences were from the same sample and 100% identical (P. Zhou et al., 2020)? If it was just a name change, why no acknowledgement of this in their 2020 paper describing RaTG13 (Bengston, 2020)? These strange and unscientific actions have obscured the origins of the closest viral relatives of SARS-CoV-2, viruses that are suspected to have caused a COVID-like illness in 2012 and which may be key to understanding not just the origin of the COVID-19 pandemic but the future behaviour of SARS-CoV-2.
-- A Proposed Origin for SARS-CoV-2 and the COVID-19 Pandemic [W/Comments], by Jonathan Latham, PhD and Allison Wilson, PhD

Second, the collection details of the two strains are nearly identical: both were collected in July 2013 from a fecal swab of R. affinis bats:

RaBtCoV/4991 was collected in a mineshaft located in the Mojiang county, which is under the jurisdiction of Pu’er City:
Mojiang Hani Autonomous County is an autonomous county under the jurisdiction of Pu’er City, in the south of Yunnan Province, China.
-- Wikipedia
And Pu’er City is listed as the collection location of RaTG13 at the GISAID database, which could well be an approximation for the Mojiang mineshaft.
It is odd that in her 2020 paper on RaTG13 Shi Zhengli fails to mention RaBtCoV/4991 or cite her 2016 paper about its discovery, for which she is listed as the one who “designed and coordinated the study”. It is not like RaBtCoV/4991 was forgotten by her group, as it is mentioned in their 2019 paper, where it is included in a phylogenetic tree of other coronaviruses:

Sampling map (A) and phylogenetic analysis of CoVs detected in Rhinolophus bats (B). A total of 19 provinces (indicated in gray) in China were involved. 1. Beijing (BJ), 2. Chongquing (CA); 3. Fujian (FJ); 4. Gansu (GS); 5. Guangdong (GD); 6. Guangxi (BX); 7. Guizhou (GZ); 8. Hainan (HaN); 9. Hebei (HeB); 10. Henan (HeN); 11. Hubei (HuB); 12. Hunan (HuN); 13. Jiangsu (JS); 14. Shandong (SD); 15. Shanxi (SX); 16. Sichuan (SC); 17. Tibet (T); 18. Yunnan (YN); and 19. Zhejiang (ZJ). The partial sequences of RdRp gene (327-bp) of CoVs detected in Rhinolophus bats were aligned with those of published represenative CoV strains. The tree was constructed by the maximum-likelihood method with bootstrap values determined with 1000 replicates. The scale bar indicates the estimated number of substitutions per 10 nucleotides. Filled triangles indicate the CoVs published previously by our lab (KU343197, KP876536, KP876544, MF094687, KP876546, KY417143, FJ588686) [15.18.40.41], filled diamonds indicate CoVs detected in this study. Putative novel alphaCoVs are labeled in green. BtCoV/Rh/YN2012 detected in Guangdong and Yunnan province in this study are in bold. FIPV, Feline infectious peritonitis virus; PEDV, procine epidemic diarrhea virus; MHV, mouse hepatitis virus. Other abbreviations are defined as those in the text. Numbers in parentheses indicate numbers of sequences sharing >97% identity.
I doubt that RaBtCoV/4991’s place in that tree was determined based solely on a 370-bp fragment, so I would think that by early 2019, Shi Zhengli’s group would have sequenced its full genome.
Intriguingly, both pangolin-2017 and pangolin-2019 genomes are also very close in this stretch of the RdRp gene, and CoV2 and pangolin-2019 share a few common mutations not found in RaTG13:

But let’s put this topic aside for now and get back to the story of Shi Zhengli’s famous 2013 Nature paper.
- admin
- Site Admin
- Posts: 40066
- Joined: Thu Aug 01, 2013 5:21 am
Re: U.S. government gave $3.7 million grant to Wuhan lab at
Part 2 of 3
“Wuhan-1”
In that paper, Shi Zhengli’s group also reported that by culturing the isolated samples in monkey Vero cells, they managed to isolate a live virus that was almost identical to the Rs3367 strain. The authors named their creation WIV1 (where WIV stands for Wuhan Institute of Virology):
Let’s compare RaTG13 with Rs3367 and RsSHC014:
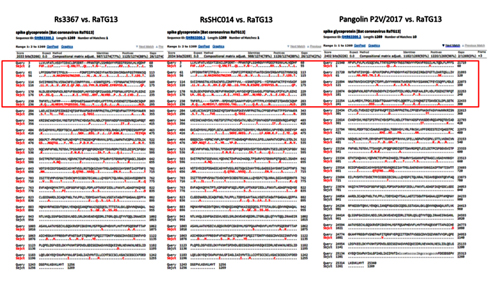
As we can see, the spike proteins of these strains are not only 13 amino acids shorter than that of RaTG13, but they also differ in the first quarter of the protein quite substantially. By the way, it is curious that the spike proteins of Rs3367 (aka WIV1) and RsSCH014 are almost identical, and differ only in the RBD region (right sequence below). Almost like CoV2 and RaTG13 (not counting the furin insert):

Could researchers, having received coronavirus samples from pangolins that were intercepted by customs in March 2019, then want to check whether the RBM in pangolin strains can bind to the human ACE2 receptor? And could such researchers also decide to throw an extra furin site in the mix?
Theoretically, of course, they could. From a technical standpoint, it is almost routine for virologists to conduct such experiments. A reasonable question might be: why use RaTG13 as a backbone, and not, say, the tried and true WIV1? Well, it doesn’t have to be either-or: maybe a chimera with WIV1 was also tested. But in parallel, they might have decided to simulate recombination of the pangolin virus with the bat strain closest to it — after all, RaTG13 is much closer to the pangolin strains than WIV1: its spike protein is closer to them both phylogenetically and structurally — it even matches them in length, while the proteins of WIV1/Rs3367 and RsSHC014 are 13 amino acids shorter. Also, the QTQTNS mutation common to RaTG13 and pangolin-2019 (MP789) just before the protease site could not have gone unnoticed by coronavirus experts.
Other Yunnan Strains
In 2011, other researchers had also found samples of coronaviruses from the Yunnan Rhinolophus affinis. The strain LYRa11 seemed to me the most interesting:
But it is also quite distant from RaTG13, and much closer to Rs3367 (that’s the strain that shares 96% with the first SARS-CoV):

But RaTG13, isolated from the same Rhinolophus affinis bats as LYRa11, looks the least like it (left sequence comparison).
Finally, another Yunnan strain (ingenuously named Yunnan2011), isolated in 2011 from another subspecies of horseshoe bats, Rhinolophus pusillus, is even less similar to RaTG13 than LYRa11:
Between themselves, Yunnan2011 and LYRa11 (the right sequence above) are not particularly similar, apart from the highly conserved S2 region. By the way, what’s up with the differing naming conventions for these strains? Sometimes they fully spell out the year, sometimes partially, yet other times not at all (Rs3367). The carrier species sometimes leads (RaTG13), sometimes follows (LYRa11). And what do TG, LY or SHC stand for? Initials of the person sequencing the genome?
Anyways, let’s move on from viral archeology to viral engineering, namely transplanting key areas of the spike protein between species and other gain-of-function (GOF) experiments.
1999: First Chimeric Coronavirus
If you think that all of the gain-of-function coronavirus research into what exactly allows coronaviruses to jump from one species to another began in response to the first SARS outbreak in 2002, you’d be mistaken. Virologists experimented with chimeric coronaviruses long before that. Here, for example, is a 1999 paper from the Dutch group of Peter Rottier from Utrecht University with a revealing title Retargeting of Coronavirus by Substitution of the Spike Glycoprotein Ectodomain: Crossing the Host Cell Species Barrier:
By the way, Shi Zhengli seems to have worked under the supervision of Peter Rottier in Utrecht for a time. At least in 2005, she co-authored a joint paper where Utrecht was listed as her affiliation (but her current address was listed at Shanghai Institute). That article itself is quite curious — in it the authors investigated what exactly allows viruses to expand their species tropism:
It is interesting that the furin site in that virus (SRRAHR | SV) is similar to the site in CoV2 (SPRRAR | SV), although in CoV2 it is cut more efficiently due to dual arginines (this is what makes it a polybasic site, i.e. it has multiple basic amino acids in a row in the RxxR sequence):
But what is especially curious is that the mutations that allowed the virus to “expand its horizons” occurred not in animals, but in vitro. Moreover, it seems, they happened pretty quickly:
Skipping ahead, I’ll just mention that there were other groups that used in vitro mutagenesis to increase the virulence of coronaviruses, for example, MERS:
Moreover, their mutations arose after just several passages (rounds of cell culture reproduction):
(F) Schematic of single and double mutation emergence in MERS-CoV spike over different passages.
(G) Location of mutations within MERS-CoV spike.
But those experiments occurred much later. In the meantime, let’s go back to 2002 — BEFORE the outbreak of the first SARS-CoV.
Ralph “Trailblazer” Baric
Ralph Baric is a legend in coronavirology. He is a trailblazer of synthetic genomic manipulation techniques. Back in 2002, he published a breakthrough work, which marked a milestone in both the study of various mechanisms of natural viruses and in gain-of-function research. In their paper, the Baric group described creating a synthetic clone of a natural murine coronavirus:
In essence, the authors have “translated” the RNA virus into the language of DNA (using reverse transcriptase), which enabled them to manipulate its genome with the help of existing genetic engineering tools. Having created 7 such cDNA provirus segments, the authors then stitched them together “seamlessly” (i.e. without introducing any new, even silent mutations, including new restrictase sites), after which they transcribed their construct back into RNA, which was then translated into virus particles in other cells.
SARS-2003
Just a few weeks after the publication of the above work, the first SARS-CoV epidemic broke out. The Baric group sprang into action. By summer of 2003, they have submitted a paper on synthetically recreating SARS-CoV:
The speed of the Baric group illustrates how quickly a qualified team of virologists can create a synthetic clone from a natural virus, and therefore make genetic modifications to it. Moreover, that was back in 2003. Today, a qualified laboratory can repeat those steps in a matter of weeks.
In fact, two just did: the Swiss have created a synthetic clone of CoV2 in under a month, while it took the Galveston BSL4 lab less than 2 months to do so.
SARS-2006
Baric was the first, but far from the last. Genetic engineering developed by leaps and bounds, creating newer and better tools. Other groups explored alternative synthetic virology techniques. For example, in 2006, Spanish researchers followed in Baric’s footsteps, also creating a synthetic SARS clone, but using an alternative approach (bacterial artificial chromosome):
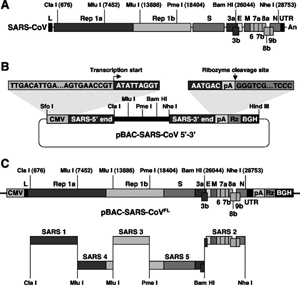
Strategy to assemble a SARS-CoV infectious cDNA clone as a BAC.
(A) Genetic structure of the SARS-CoV Urbani strain genome. Relevant restriction sites used for the assembly of the full-length cDNA clone are indicated. Numbers in parentheses indicate the genomic positions of the first nucleotide of the restriction endonuclease recognition sequence. Letters and numbers indicate the viral genes. L, leader sequence; UTR, untranslated region; An, poly(A) tail. (B) Construction of pBAC-SARS-CoV 5′-3′. After the selection of appropriate restriction sites, the intermediate plasmid pBAC-SARS-CoV 5′-3′ was constructed as the backbone for assembling the infectious cDNA clone. This plasmid includes the first 681 nt of the genome under the control of the CMV promoter, a multiple-cloning site containing the restriction sites selected for the final assembly of the infectious clone, and the last 975 nt of the genome, followed by a synthetic poly(A) tail (pA), the hepatitis delta virus ribozyme (Rz), and the bovine growth hormone termination and polyadenylation sequences (BGH). All these elements were precisely joined by overlapping PCR. The CMV promoter transcription start and the ribozyme cleavage site are shown. © Schematic diagram showing the five-step cloning strategy used for the assembly of the SARS-CoV full-length cDNA clone. The five overlapping cDNA fragments, named SARS 1 to SARS 5, were sequentially cloned into the plasmid pBAC-SARS-CoV 5′-3′ to generate the plasmid pBAC-SARS-CoVFL. Relevant restriction sites are indicated. The labels are as described for panel A.
True, they didn’t do it as elegantly as Baric, as their final assembly of the synthetic virus included their added restriction enzyme sites, while Baric learned to combine fragments “seamlessly”. But this is a minor point, the Spanish approach is just as robust — in 2013, with its help, the same authors had created a synthetic clone of MERS, and in 2015 their technique was included in a coronavirus textbook (chapter 13).
Wuhan 2007
Let’s get back to 2007. That is when the Shi Zhengli group joined the synthetic virology race with a study of the spike protein of human and bat coronaviruses, trying to determine what exactly is responsible for the ability to skip from one species to another:
That is, the authors inserted different segments from the human SARS-CoV spike protein into the spike protein of the bat virus. Here is their conclusion:
At the same time, they tried to replace shorter fragments, including just the RBM:
Given that the above was written in 2007, I think today it will not be difficult for even a novice virologist to replace the RBM of one virus by an RBM from another.
Chimera-2015
In light of the above experiments, it is not very clear what caused the uproar that followed probably the most famous gain-of-function virology paper. I am referring to the joint 2015 work of Shi Zhengli and Ralph Baric, in which they created a synthetic chimeric virus:
To me, the authors followed a familiar path: they took the spike-like protein from RsSHC014, which Shi Zhengli isolated from Yunnan bats in 2011, and inserted it into a murine-adapted variant of SARS-CoV for subsequent in vivo experiments. They also tested it in human cells, and almost as an aside created a recombinant clone of the same RsSHC014 strain:
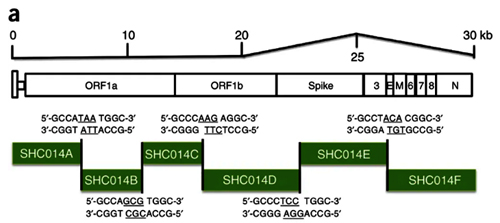
(a) Schematic of the SHC014-CoV molecular clone, which was synthesized as six contiguous cDNAs (designated SHC014A, SHC014B, SHC014C, SHC014D, SHC014E and SHC014F) flanked by unique BglI sites that allowed for directed assembly of the full-length cDNA expressing open reading frames (for 1a, 1b, spike, 3, envelope, matrix, 6–8 and nucleocapsid). Underlined nucleotides represent the overhang sequences formed after restriction enzyme cleavage.
The researchers also uncovered that it was not only the binding of spike protein to the receptor that determined the virus’s potential for transition from one animal species to another, because the SHC014-MA15 chimera was more virulent than SHC014 itself, even in human cells:
I especially want to highlight the spike processivity in the quote, because this is not the first time that virologists have mentioned that the ability of a spike protein to be cleaved by proteases (including furin) can have an impact on virulence.
That’s all I have to say about that paper. As a curiosity here is a common photo of its key authors, which was taken in Wuhan, in October 2018. Fittingly, Ralph Bariс and Shi Zhengli are front and center. I call this photo “The Wuhan Clan”. (Sorry, couldn’t resist).

Murine SARS-2007
One quick aside regarding the “murine virus MA15” from the above paper. That was not some kind of natural murine coronavirus, as one might think. It was a laboratory-modified human SARS-CoV, which back in 2007 the Baric group — possibly in competition with the Shi Zhengli group (remember their article from 2007) — turned into a real beast. To do this, they first iteratively “improved” it in mice, and when after several iterations it became maximally “effective”, they reproduced the observed mutations in a synthetic clone, and once again checked that it really does have increased virulence and lethality:
Baric-2008
Here is another example of the potential scientific rivalry between the Baric and Shi Zhengli groups. In 2008, the Baric group took the Bat-SCoV strain and replaced its RBD with an RBD from human SARS. That is, they essentially reproduced the work of Shi Zhengli’s group from 2007, except they didn’t limit themselves to pseudo-viruses, but created a real chimeric virus:
(B) Schematic representation showing organization of the SARS-CoV and Bat-SCoV Spike proteins. The engineered Spike proteins are pictured below with the virus name to the left. Bat-SRBD includes all of the Bat-SCoV Spike sequence except that the Bat-SCoV RBD (Bat-SCoV amino acid 323–505) is replaced with the SARS-CoV RBD (amino acid 319–518) (GenBank accession no. FJ211860). Bat-SRBD-MA includes the MA15 Spike RBD change at SARS-CoV aa Y436H. Bat-SRBM includes the minimal 13 SARS-CoV residues critical for ACE2 contact, resulting in a chimeric RBD of Bat-SCoV amino acid 323I-429T and SARS-CoV amino acid 426R-518D. Bat-Hinge is Bat-SRBM sequence, with Bat-SCoV amino acid 392L-397E replaced with SARS-CoV amino acid 388V-393D. Bat-F includes nt 1–24057 of SARS-CoV (to Spike amino acid 855), with the remaining 3′ sequence from Bat-SCoV. To the right of the schematic representations, observation of transcript activity and approximate stock titers at passage 1 (P1) are indicated. ND indicates no infectious virus detected by plaque assay.
Baric-2016
The Baric group does seem to have its share of similar papers. For example, in 2016, they essentially repeated their collaboration with Shi Zhengli from 2015 to create a chimeric virus, only this time they inserted a spike protein segment into their mouse-adapted SARS not from RsSCH014, but from another strain Shi Zhengli found in Yunnan — its close relative Rs3367. Or, to be exact, from WIV1 — the laboratory clone of Rs3367 isolated at the Wuhan Institute of Virology in 2013:
To me, the 2016 paper looks a lot like the 2015 one. Moreover, its rationale is not very clear to me: after all, WIV1/Rs3367 already shared 96% of their genome with SARS-CoV. So I am not sure why one would want to insert a spike protein from its closest relative back into SARS-CoV. Maybe just because they could. In this light, the title of the article acquires a certain duality: SARS-like WIV1-CoV poised for human emergence.
Oh, and I am not sure how in 2015 Baric was granted a patent for the creation of “chimeric coronavirus spike proteins”, given all that he and Shi Zhengli previously disclosed in their papers long before 2015.
Baric-1990
Just so you appreciate how long Ralph Baric has been at this game — he was designing recombinant coronaviruses way before there were any DNA sequencing machines or other modern tools of genetic engineering. Here is his paper on the creation of “temperature mutants” from mouse coronavirus from 1990:
So Dr. Baric has been creating mutant viruses for over 30 years.
Wuhan-2017
The Shi Zhengli group has also not been idle since the famous 2015 paper. In 2017, they published a paper where they reported creating not one but 8 chimeric viruses — all made using transplanted RBDs from bat SARS-like viruses which they collected over a span of 5 years from the very cave around Kunming, Yunnan Province, where Shi Zhengli originally found Rs3367 and RsSCH014.
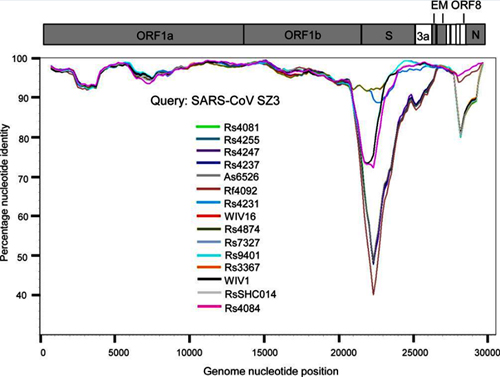
Similarity plot based on the full-length genome sequence of civet SARS CoV SZ3.
Full-length genome sequences of all SARSr-CoV detected in bats from the cave investigated in this study were used as reference sequences. The analysis was performed with the Kimura model, a window size of 1500 base pairs and a step size of 150 base pairs.
The authors then checked if their chimeras can infect human cells, and this time they used a live synthetic virus, rather than not pseudo-typed HIV constructs as before:
Baric-2019
Ralph Baric also showed no signs of slowing down. At the end of October 2019, his group submitted for publication another paper on the importance of spike protein protease cleavage (remember the furin site?) to crossing the “barrier to zoonotic infection” by coronaviruses:
As I recall the spirit of scientific competition between the groups of Baric and Shi Zhengli, I can’t help but wonder whether someone was conducting similar research in the Wuhan lab in 2019.
Gain-of-Function: Risky Business
Many people who first learn about the above research ask a very valid question: “But why?” Why do scientists create chimeric killer viruses? The politically correct answer is to develop preventive measures (drugs or vaccines) from possible natural chimeras and to understand the risks of their occurrence. Here, in fact, is what Baric, Shi Zhengli, and co-authors themselves wrote on this subject in their famous 2015 paper:
Were these words prophetic? At the end of 2014, the United States introduced a moratorium on state financing of such gain-of-function studies, but it was shortly canceled (in 2017). In China, no moratorium on such studies was introduced, on the contrary, they went full steam ahead with creating new “super labs” of the highest biosafety level (BSL-4), as in 2017 in Wuhan:

To be clear, the Wuhan lab was allowed to work with coronaviruses even before 2017, as these viruses only required a BSL-3 rating which the Wuhan Institute of Virology had. But their aspirations to obtain BSL-4 made a lot of people uneasy, including fellow researchers:
Interestingly, in addition to Wuhan, the Chinese government planned to open a new BSL-4 lab in Kunming, with an eye to testing vaccines on primates. As you might recall, Kunming is not only the capital of Yunnan, but it is also where Shi Zhengli found the strains Rs3367 and RsSHC014 in nearby caves. By the way, primate testing was mentioned by Baric and Shi Zhengli as possible next steps for the development of preventive vaccines against potential future outbreaks of coronaviruses in their famous 2015 paper:
Maybe by 2019 the creation and testing of potential vaccines against various SARS-like coronaviruses was already in full swing.
“Wuhan-1”
In that paper, Shi Zhengli’s group also reported that by culturing the isolated samples in monkey Vero cells, they managed to isolate a live virus that was almost identical to the Rs3367 strain. The authors named their creation WIV1 (where WIV stands for Wuhan Institute of Virology):
Most importantly, we report the first recorded isolation of a live SL-CoV (bat SL-CoV-WIV1) from bat faecal samples in Vero E6 cells, which has typical coronavirus morphology, 99.9% sequence identity to Rs3367 and uses ACE2 from humans, civets and Chinese horseshoe bats for cell entry. Preliminary in vitro testing indicates that WIV1 also has a broad species tropism.
Let’s compare RaTG13 with Rs3367 and RsSHC014:

As we can see, the spike proteins of these strains are not only 13 amino acids shorter than that of RaTG13, but they also differ in the first quarter of the protein quite substantially. By the way, it is curious that the spike proteins of Rs3367 (aka WIV1) and RsSCH014 are almost identical, and differ only in the RBD region (right sequence below). Almost like CoV2 and RaTG13 (not counting the furin insert):

Could researchers, having received coronavirus samples from pangolins that were intercepted by customs in March 2019, then want to check whether the RBM in pangolin strains can bind to the human ACE2 receptor? And could such researchers also decide to throw an extra furin site in the mix?
Theoretically, of course, they could. From a technical standpoint, it is almost routine for virologists to conduct such experiments. A reasonable question might be: why use RaTG13 as a backbone, and not, say, the tried and true WIV1? Well, it doesn’t have to be either-or: maybe a chimera with WIV1 was also tested. But in parallel, they might have decided to simulate recombination of the pangolin virus with the bat strain closest to it — after all, RaTG13 is much closer to the pangolin strains than WIV1: its spike protein is closer to them both phylogenetically and structurally — it even matches them in length, while the proteins of WIV1/Rs3367 and RsSHC014 are 13 amino acids shorter. Also, the QTQTNS mutation common to RaTG13 and pangolin-2019 (MP789) just before the protease site could not have gone unnoticed by coronavirus experts.
Other Yunnan Strains
In 2011, other researchers had also found samples of coronaviruses from the Yunnan Rhinolophus affinis. The strain LYRa11 seemed to me the most interesting:

But it is also quite distant from RaTG13, and much closer to Rs3367 (that’s the strain that shares 96% with the first SARS-CoV):

But RaTG13, isolated from the same Rhinolophus affinis bats as LYRa11, looks the least like it (left sequence comparison).
Finally, another Yunnan strain (ingenuously named Yunnan2011), isolated in 2011 from another subspecies of horseshoe bats, Rhinolophus pusillus, is even less similar to RaTG13 than LYRa11:

Between themselves, Yunnan2011 and LYRa11 (the right sequence above) are not particularly similar, apart from the highly conserved S2 region. By the way, what’s up with the differing naming conventions for these strains? Sometimes they fully spell out the year, sometimes partially, yet other times not at all (Rs3367). The carrier species sometimes leads (RaTG13), sometimes follows (LYRa11). And what do TG, LY or SHC stand for? Initials of the person sequencing the genome?
Anyways, let’s move on from viral archeology to viral engineering, namely transplanting key areas of the spike protein between species and other gain-of-function (GOF) experiments.
1999: First Chimeric Coronavirus
If you think that all of the gain-of-function coronavirus research into what exactly allows coronaviruses to jump from one species to another began in response to the first SARS outbreak in 2002, you’d be mistaken. Virologists experimented with chimeric coronaviruses long before that. Here, for example, is a 1999 paper from the Dutch group of Peter Rottier from Utrecht University with a revealing title Retargeting of Coronavirus by Substitution of the Spike Glycoprotein Ectodomain: Crossing the Host Cell Species Barrier:
Using targeted RNA recombination, we constructed a mutant of the coronavirus mouse hepatitis virus (MHV) in which the ectodomain of the spike glycoprotein (S) was replaced with the highly divergent ectodomain of the S protein of feline infectious peritonitis virus. The resulting chimeric virus, designated fMHV, acquired the ability to infect feline cells and simultaneously lost the ability to infect murine cells in tissue culture.
By the way, Shi Zhengli seems to have worked under the supervision of Peter Rottier in Utrecht for a time. At least in 2005, she co-authored a joint paper where Utrecht was listed as her affiliation (but her current address was listed at Shanghai Institute). That article itself is quite curious — in it the authors investigated what exactly allows viruses to expand their species tropism:
Only a relatively few mutations in its spike protein allow the murine coronavirus to switch from a murine-restricted tropism to an extended host range by being passaged in vitro. One such virus that we studied had acquired two putative heparan sulfate-binding sites while preserving another site in the furin-cleavage motif. The adaptation of the virus through the use of heparan sulfate as an attachment/entry receptor was demonstrated by increased heparin binding as well as by inhibition of infection through treatment of cells and the virus with heparinase and heparin, respectively.
It is interesting that the furin site in that virus (SRRAHR | SV) is similar to the site in CoV2 (SPRRAR | SV), although in CoV2 it is cut more efficiently due to dual arginines (this is what makes it a polybasic site, i.e. it has multiple basic amino acids in a row in the RxxR sequence):

But what is especially curious is that the mutations that allowed the virus to “expand its horizons” occurred not in animals, but in vitro. Moreover, it seems, they happened pretty quickly:
MHV/pi23, a virus obtained after 23 of the 600 passages that resulted in MHV/BHK, also contains a putative HS-binding site in the S1 domain at the same position as in MHV/BHK, albeit as a smaller insertion, while it lacks the putative HS-binding site immediately upstream of the fusion peptide. MHV/pi23 does infect nonmurine cells to some extent but much less efficiently than MHV/BHK. In addition to the multiple HS-binding sites, however, mutations found in other parts of the S protein, such as the HR1 domain and the putative fusion peptide (Fig. 1), might also contribute to the efficient entry into nonmurine cells. We are currently in the process of determining the S protein mutations that are required for the extended host range phenotype.
Skipping ahead, I’ll just mention that there were other groups that used in vitro mutagenesis to increase the virulence of coronaviruses, for example, MERS:
To better understand the species adaptability of MERS-CoV, we identified a suboptimal species-derived variant of DPP4 to study viral adaption. Passaging virus on cells expressing this DPP4 variant led to accumulation of mutations in the viral spike which increased replication.
Moreover, their mutations arose after just several passages (rounds of cell culture reproduction):

(F) Schematic of single and double mutation emergence in MERS-CoV spike over different passages.
(G) Location of mutations within MERS-CoV spike.
But those experiments occurred much later. In the meantime, let’s go back to 2002 — BEFORE the outbreak of the first SARS-CoV.
Ralph “Trailblazer” Baric
Ralph Baric is a legend in coronavirology. He is a trailblazer of synthetic genomic manipulation techniques. Back in 2002, he published a breakthrough work, which marked a milestone in both the study of various mechanisms of natural viruses and in gain-of-function research. In their paper, the Baric group described creating a synthetic clone of a natural murine coronavirus:
A novel method was developed to assemble a full-length infectious cDNA of the group II coronavirus mouse hepatitis virus strain A59 (MHV-A59). Seven contiguous cDNA clones that spanned the 31.5-kb MHV genome were isolated. The ends of the cDNAs were engineered with unique junctions and assembled with only the adjacent cDNA subclones, resulting in an intact MHV-A59 cDNA construct of ∼31.5 kb in length. The interconnecting restriction site junctions that are located at the ends of each cDNA are systematically removed during the assembly of the complete full-length cDNA product, allowing reassembly without the introduction of nucleotide changes… The method has the potential to be used to construct viral, microbial, or eukaryotic genomes approaching several million base pairs in length and used to insert restriction sites at any given nucleotide in a microbial genome.

In essence, the authors have “translated” the RNA virus into the language of DNA (using reverse transcriptase), which enabled them to manipulate its genome with the help of existing genetic engineering tools. Having created 7 such cDNA provirus segments, the authors then stitched them together “seamlessly” (i.e. without introducing any new, even silent mutations, including new restrictase sites), after which they transcribed their construct back into RNA, which was then translated into virus particles in other cells.
A reverse transcriptase (RT) is an enzyme used to generate complementary DNA (cDNA) from an RNA template, a process termed reverse transcription. Reverse transcriptases are used by certain viruses such as HIV and the hepatitis B virus to replicate their genomes, by retrotransposon mobile genetic elements to proliferate within the host genome, and by eukaryotic cells to extend the telomeres at the ends of their linear chromosomes.
-- Reverse transcriptase, by Wikipedia
SARS-2003
Just a few weeks after the publication of the above work, the first SARS-CoV epidemic broke out. The Baric group sprang into action. By summer of 2003, they have submitted a paper on synthetically recreating SARS-CoV:
Using a panel of contiguous cDNAs that span the entire genome, we have assembled a full-length cDNA of the SARS-CoV Urbani strain, and have rescued molecularly cloned SARS viruses (infectious clone SARS-CoV) that contained the expected marker mutations inserted into the component clones. Recombinant viruses replicated as efficiently as WT virus and both were inhibited by treatment with the cysteine proteinase inhibitor… Availability of a SARS-CoV full-length cDNA provides a template for manipulation of the viral genome, allowing for the rapid and rational development and testing of candidate vaccines and therapeutics against this important human pathogen.

The speed of the Baric group illustrates how quickly a qualified team of virologists can create a synthetic clone from a natural virus, and therefore make genetic modifications to it. Moreover, that was back in 2003. Today, a qualified laboratory can repeat those steps in a matter of weeks.
In fact, two just did: the Swiss have created a synthetic clone of CoV2 in under a month, while it took the Galveston BSL4 lab less than 2 months to do so.
SARS-2006
Baric was the first, but far from the last. Genetic engineering developed by leaps and bounds, creating newer and better tools. Other groups explored alternative synthetic virology techniques. For example, in 2006, Spanish researchers followed in Baric’s footsteps, also creating a synthetic SARS clone, but using an alternative approach (bacterial artificial chromosome):
The engineering of a full-length infectious cDNA clone and a functional replicon of the severe acute respiratory syndrome coronavirus (SARS-CoV) Urbani strain as bacterial artificial chromosomes (BACs) is described in this study. In this system, the viral RNA was expressed in the cell nucleus under the control of the cytomegalovirus promoter and further amplified in the cytoplasm by the viral replicase. Both the infectious clone and the replicon were fully stable in Escherichia coli.
…
The assembled SARS-CoV infectious cDNA clone was fully stable during its propagation in E. coli DH10B cells for more than 200 generations, considerably facilitating the genetic manipulation of the viral genome (data not shown). The detailed cloning strategy, plasmid maps, and sequences are available upon request.

Strategy to assemble a SARS-CoV infectious cDNA clone as a BAC.
(A) Genetic structure of the SARS-CoV Urbani strain genome. Relevant restriction sites used for the assembly of the full-length cDNA clone are indicated. Numbers in parentheses indicate the genomic positions of the first nucleotide of the restriction endonuclease recognition sequence. Letters and numbers indicate the viral genes. L, leader sequence; UTR, untranslated region; An, poly(A) tail. (B) Construction of pBAC-SARS-CoV 5′-3′. After the selection of appropriate restriction sites, the intermediate plasmid pBAC-SARS-CoV 5′-3′ was constructed as the backbone for assembling the infectious cDNA clone. This plasmid includes the first 681 nt of the genome under the control of the CMV promoter, a multiple-cloning site containing the restriction sites selected for the final assembly of the infectious clone, and the last 975 nt of the genome, followed by a synthetic poly(A) tail (pA), the hepatitis delta virus ribozyme (Rz), and the bovine growth hormone termination and polyadenylation sequences (BGH). All these elements were precisely joined by overlapping PCR. The CMV promoter transcription start and the ribozyme cleavage site are shown. © Schematic diagram showing the five-step cloning strategy used for the assembly of the SARS-CoV full-length cDNA clone. The five overlapping cDNA fragments, named SARS 1 to SARS 5, were sequentially cloned into the plasmid pBAC-SARS-CoV 5′-3′ to generate the plasmid pBAC-SARS-CoVFL. Relevant restriction sites are indicated. The labels are as described for panel A.
True, they didn’t do it as elegantly as Baric, as their final assembly of the synthetic virus included their added restriction enzyme sites, while Baric learned to combine fragments “seamlessly”. But this is a minor point, the Spanish approach is just as robust — in 2013, with its help, the same authors had created a synthetic clone of MERS, and in 2015 their technique was included in a coronavirus textbook (chapter 13).
Wuhan 2007
Let’s get back to 2007. That is when the Shi Zhengli group joined the synthetic virology race with a study of the spike protein of human and bat coronaviruses, trying to determine what exactly is responsible for the ability to skip from one species to another:
A series of S chimeras was constructed by inserting different sequences of the SARS-CoV S into the SL-CoV S backbone.
That is, the authors inserted different segments from the human SARS-CoV spike protein into the spike protein of the bat virus. Here is their conclusion:
From these results, it was deduced that the region from aa 310 to 518 of BJ01-S was necessary and sufficient to convert Rp3-S into a huACE2-binding molecule.
At the same time, they tried to replace shorter fragments, including just the RBM:
.For introduction of the RBM of SARS-CoV S into the SL-CoV S, the coding region from aa 424 to 494 of BJ01-S was used to replace the corresponding regions of Rp3-S, resulting in a chimeric S (CS) gene designated CS424–494
Given that the above was written in 2007, I think today it will not be difficult for even a novice virologist to replace the RBM of one virus by an RBM from another.
Chimera-2015
In light of the above experiments, it is not very clear what caused the uproar that followed probably the most famous gain-of-function virology paper. I am referring to the joint 2015 work of Shi Zhengli and Ralph Baric, in which they created a synthetic chimeric virus:
Using the SARS-CoV reverse genetics system, we generated and characterized a chimeric virus expressing the spike of bat coronavirus SHC014 in a mouse-adapted SARS-CoV backbone. The results indicate that group 2b viruses encoding the SHC014 spike in a wild-type backbone can efficiently use multiple orthologs of the SARS receptor human angiotensin converting enzyme II (ACE2), replicate efficiently in primary human airway cells and achieve in vitro titers equivalent to epidemic strains of SARS-CoV. Additionally, in vivo experiments demonstrate replication of the chimeric virus in mouse lung with notable pathogenesis. Evaluation of available SARS-based immune-therapeutic and prophylactic modalities revealed poor efficacy; both monoclonal antibody and vaccine approaches failed to neutralize and protect from infection with CoVs using the novel spike protein. On the basis of these findings, we synthetically re-derived an infectious full-length SHC014 recombinant virus and demonstrate robust viral replication both in vitro and in vivo.
To me, the authors followed a familiar path: they took the spike-like protein from RsSHC014, which Shi Zhengli isolated from Yunnan bats in 2011, and inserted it into a murine-adapted variant of SARS-CoV for subsequent in vivo experiments. They also tested it in human cells, and almost as an aside created a recombinant clone of the same RsSHC014 strain:

(a) Schematic of the SHC014-CoV molecular clone, which was synthesized as six contiguous cDNAs (designated SHC014A, SHC014B, SHC014C, SHC014D, SHC014E and SHC014F) flanked by unique BglI sites that allowed for directed assembly of the full-length cDNA expressing open reading frames (for 1a, 1b, spike, 3, envelope, matrix, 6–8 and nucleocapsid). Underlined nucleotides represent the overhang sequences formed after restriction enzyme cleavage.
The researchers also uncovered that it was not only the binding of spike protein to the receptor that determined the virus’s potential for transition from one animal species to another, because the SHC014-MA15 chimera was more virulent than SHC014 itself, even in human cells:
Notably, differential tropism in the lung as compared to that with SARS-MA15 and attenuation of full-length SHC014-CoV in [human epithelial airway cell] cultures relative to SARS-CoV Urbani suggest that factors beyond ACE2 binding — including spike processivity, receptor bio-availability or antagonism of the host immune responses — may contribute to emergence.
I especially want to highlight the spike processivity in the quote, because this is not the first time that virologists have mentioned that the ability of a spike protein to be cleaved by proteases (including furin) can have an impact on virulence.
That’s all I have to say about that paper. As a curiosity here is a common photo of its key authors, which was taken in Wuhan, in October 2018. Fittingly, Ralph Bariс and Shi Zhengli are front and center. I call this photo “The Wuhan Clan”. (Sorry, couldn’t resist).

Murine SARS-2007
One quick aside regarding the “murine virus MA15” from the above paper. That was not some kind of natural murine coronavirus, as one might think. It was a laboratory-modified human SARS-CoV, which back in 2007 the Baric group — possibly in competition with the Shi Zhengli group (remember their article from 2007) — turned into a real beast. To do this, they first iteratively “improved” it in mice, and when after several iterations it became maximally “effective”, they reproduced the observed mutations in a synthetic clone, and once again checked that it really does have increased virulence and lethality:
We adapted the SARS-CoV (Urbani strain) by serial passage in the respiratory tract of young BALB/c mice. Fifteen passages resulted in a virus (MA15) that is lethal for mice following intranasal inoculation. Lethality is preceded by rapid and high titer viral replication in lungs, viremia, and dissemination of virus to extrapulmonary sites accompanied by lymphopenia, neutrophilia, and pathological changes in the lungs. Abundant viral antigen is extensively distributed in bronchial epithelial cells and alveolar pneumocytes, and necrotic cellular debris is present in airways and alveoli, with only mild and focal pneumonitis. These observations suggest that mice infected with MA15 die from an overwhelming viral infection with extensive, virally mediated destruction of pneumocytes and ciliated epithelial cells. The MA15 virus has six coding mutations associated with adaptation and increased virulence; when introduced into a recombinant SARS-CoV, these mutations result in a highly virulent and lethal virus (rMA15), duplicating the phenotype of the biologically derived MA15 virus. Intranasal inoculation with MA15 reproduces many aspects of disease seen in severe human cases of SARS.
Baric-2008
Here is another example of the potential scientific rivalry between the Baric and Shi Zhengli groups. In 2008, the Baric group took the Bat-SCoV strain and replaced its RBD with an RBD from human SARS. That is, they essentially reproduced the work of Shi Zhengli’s group from 2007, except they didn’t limit themselves to pseudo-viruses, but created a real chimeric virus:
As compared to a live virus, pseudoviruses, which can be either naturally produced during an infection or artificially in a laboratory for research purposes, contain fragments of host-cell DNA without containing any of the nucleic acid components of the infectious virus to which they are related.
The modified genetic material of pseudoviruses prevents these particles from producing viral surface proteins on their own unless an additional plasmid or stable cell line that expresses such proteins are made available to the pseudovirus.
-- What is a Pseudovirus?, by News Medical Life Sciences
Here, we report the design, synthesis, and recovery of the largest synthetic replicating life form, a 29.7-kb bat severe acute respiratory syndrome (SARS)-like coronavirus (Bat-SCoV), a likely progenitor to the SARS-CoV epidemic.
…
To test whether the RBDs of Bat-SCoV and SARS-CoV were interchangeable, we replaced the Bat-SCoV RBD (amino acid 323–505) with the SARS-CoV RBD (amino acid 319–518) (27, 28) (GenBank accession no. FJ211860), simulating a theoretical recombination event that might occur during mixed infection in vivo (Fig. 1B).

(B) Schematic representation showing organization of the SARS-CoV and Bat-SCoV Spike proteins. The engineered Spike proteins are pictured below with the virus name to the left. Bat-SRBD includes all of the Bat-SCoV Spike sequence except that the Bat-SCoV RBD (Bat-SCoV amino acid 323–505) is replaced with the SARS-CoV RBD (amino acid 319–518) (GenBank accession no. FJ211860). Bat-SRBD-MA includes the MA15 Spike RBD change at SARS-CoV aa Y436H. Bat-SRBM includes the minimal 13 SARS-CoV residues critical for ACE2 contact, resulting in a chimeric RBD of Bat-SCoV amino acid 323I-429T and SARS-CoV amino acid 426R-518D. Bat-Hinge is Bat-SRBM sequence, with Bat-SCoV amino acid 392L-397E replaced with SARS-CoV amino acid 388V-393D. Bat-F includes nt 1–24057 of SARS-CoV (to Spike amino acid 855), with the remaining 3′ sequence from Bat-SCoV. To the right of the schematic representations, observation of transcript activity and approximate stock titers at passage 1 (P1) are indicated. ND indicates no infectious virus detected by plaque assay.
Baric-2016
The Baric group does seem to have its share of similar papers. For example, in 2016, they essentially repeated their collaboration with Shi Zhengli from 2015 to create a chimeric virus, only this time they inserted a spike protein segment into their mouse-adapted SARS not from RsSCH014, but from another strain Shi Zhengli found in Yunnan — its close relative Rs3367. Or, to be exact, from WIV1 — the laboratory clone of Rs3367 isolated at the Wuhan Institute of Virology in 2013:
Using the SARS-CoV infectious clone as a template (7), we designed and synthesized a full-length infectious clone of WIV1-CoV consisting of six plasmids that could be enzymatically cut, ligated together, and electroporated into cells to rescue replication competent progeny virions (Fig. S1A). In addition to the full-length clone, we also produced WIV1-CoV chimeric virus that replaced the SARS spike with the WIV1 spike within the mouse-adapted backbone (WIV1-MA15, Fig. S1B). … To confirm growth kinetics and replication, Vero cells were infected with SARS-CoV Urbani, WIV1-MA15, and WIV1-CoV.

To me, the 2016 paper looks a lot like the 2015 one. Moreover, its rationale is not very clear to me: after all, WIV1/Rs3367 already shared 96% of their genome with SARS-CoV. So I am not sure why one would want to insert a spike protein from its closest relative back into SARS-CoV. Maybe just because they could. In this light, the title of the article acquires a certain duality: SARS-like WIV1-CoV poised for human emergence.
Oh, and I am not sure how in 2015 Baric was granted a patent for the creation of “chimeric coronavirus spike proteins”, given all that he and Shi Zhengli previously disclosed in their papers long before 2015.
Baric-1990
Just so you appreciate how long Ralph Baric has been at this game — he was designing recombinant coronaviruses way before there were any DNA sequencing machines or other modern tools of genetic engineering. Here is his paper on the creation of “temperature mutants” from mouse coronavirus from 1990:
The A59 strain of mouse hepatitis virus (MHV-A59) was used throughout the course of this study. Virus was propagated and cloned three times in the continuous murine astrocytoma cell line (DBT).
…
Various combinations of [temperature sensitive] mutants were mixed and inoculated onto cells at a multiplicity of infection of 10 each.
So Dr. Baric has been creating mutant viruses for over 30 years.
Wuhan-2017
The Shi Zhengli group has also not been idle since the famous 2015 paper. In 2017, they published a paper where they reported creating not one but 8 chimeric viruses — all made using transplanted RBDs from bat SARS-like viruses which they collected over a span of 5 years from the very cave around Kunming, Yunnan Province, where Shi Zhengli originally found Rs3367 and RsSCH014.
Using the reverse genetics technique we previously developed for WIV1 [23], we constructed a group of infectious bacterial artificial chromosome (BAC) clones with the backbone of WIV1 and variants of S genes from 8 different bat SARSr-CoVs. Only the infectious clones for Rs4231 and Rs7327 led to cytopathic effects in Vero E6 cells after transfection (S7 Fig). The other six strains with deletions in the RBD region, Rf4075, Rs4081, Rs4085, Rs4235, As6526 and Rp3 (S1 Fig) failed to be rescued, as no cytopathic effects was observed and viral replication cannot be detected by immunofluorescence assay in Vero E6 cells (S7 Fig). In contrast, when Vero E6 cells were respectively infected with the two successfully rescued chimeric SARSr-CoVs, WIV1-Rs4231S and WIV1-Rs7327S, and the newly isolated Rs4874, efficient virus replication was detected in all infections (Fig 7).

Similarity plot based on the full-length genome sequence of civet SARS CoV SZ3.
Full-length genome sequences of all SARSr-CoV detected in bats from the cave investigated in this study were used as reference sequences. The analysis was performed with the Kimura model, a window size of 1500 base pairs and a step size of 150 base pairs.
The authors then checked if their chimeras can infect human cells, and this time they used a live synthetic virus, rather than not pseudo-typed HIV constructs as before:
To assess whether the three novel SARSr-CoVs can use human ACE2 as a cellular entry receptor, we conducted virus infectivity studies using HeLa cells with or without the expression of human ACE2. All viruses replicated efficiently in the human ACE2-expressing cells. The results were further confirmed by quantification of viral RNA using real-time RT-PCR (Fig 8).
Baric-2019
Ralph Baric also showed no signs of slowing down. At the end of October 2019, his group submitted for publication another paper on the importance of spike protein protease cleavage (remember the furin site?) to crossing the “barrier to zoonotic infection” by coronaviruses:
Together, these results demonstrate that protease cleavage is also the primary barrier to infection of Vero cells with HKU5-CoV. Examining further, we compared the predicted cleavage at S1/S2 border, S2’, and the endosomal cysteine protease site across MERS, PDF2180, and HKU5 spikes (Fig. 6D) (26). For the S1/S2 site, MERS, Uganda, and HKU5 maintain the RXXR cleavage motif, although the different interior amino acids may alter efficiency. For the S2’ sequence, MERS and HKU5 also retain the RXXR motif; however, the Uganda spike lacks the first arginine (SNAR), potentially impacting cleavage.
As I recall the spirit of scientific competition between the groups of Baric and Shi Zhengli, I can’t help but wonder whether someone was conducting similar research in the Wuhan lab in 2019.
Gain-of-Function: Risky Business
Many people who first learn about the above research ask a very valid question: “But why?” Why do scientists create chimeric killer viruses? The politically correct answer is to develop preventive measures (drugs or vaccines) from possible natural chimeras and to understand the risks of their occurrence. Here, in fact, is what Baric, Shi Zhengli, and co-authors themselves wrote on this subject in their famous 2015 paper:
In addition to offering preparation against future emerging viruses, this approach must be considered in the context of the US government–mandated pause on gain-of-function (GOF) studies. On the basis of previous models of emergence (Fig. 4a,b), the creation of chimeric viruses such as SHC014-MA15 was not expected to increase pathogenicity. Although SHC014-MA15 is attenuated relative to its parental mouse-adapted SARS-CoV, similar studies examining the pathogenicity of CoVs with the wild-type Urbani spike within the MA15 backbone showed no weight loss in mice and reduced viral replication. Thus, relative to the Urbani spike–MA15 CoV, SHC014-MA15 shows a gain in pathogenesis (Fig. 1). On the basis of these findings, scientific review panels may deem similar studies building chimeric viruses based on circulating strains too risky to pursue, as increased pathogenicity in mammalian models cannot be excluded. Coupled with restrictions on mouse-adapted strains and the development of monoclonal antibodies using escape mutants, research into CoV emergence and therapeutic efficacy may be severely limited moving forward. Together, these data and restrictions represent a crossroads of GOF research concerns; the potential to prepare for and mitigate future outbreaks must be weighed against the risk of creating more dangerous pathogens. In developing policies moving forward, it is important to consider the value of the data generated by these studies and whether these types of chimeric virus studies warrant further investigation versus the inherent risks involved.
Were these words prophetic? At the end of 2014, the United States introduced a moratorium on state financing of such gain-of-function studies, but it was shortly canceled (in 2017). In China, no moratorium on such studies was introduced, on the contrary, they went full steam ahead with creating new “super labs” of the highest biosafety level (BSL-4), as in 2017 in Wuhan:

To be clear, the Wuhan lab was allowed to work with coronaviruses even before 2017, as these viruses only required a BSL-3 rating which the Wuhan Institute of Virology had. But their aspirations to obtain BSL-4 made a lot of people uneasy, including fellow researchers:
Future plans include studying the pathogen that causes SARS, which also doesn’t require a BSL-4 lab, before moving on to Ebola and the West African Lassa virus, which do. Some one million Chinese people work in Africa; the country needs to be ready for any eventuality, says Yuan. “Viruses don’t know borders.”
…
The plan to expand into a network heightens such concerns. One BSL-4 lab in Harbin is already awaiting accreditation; the next two are expected to be in Beijing and Kunming, the latter focused on using monkey models to study disease.
Lina says that China’s size justifies this scale, and that the opportunity to combine BSL-4 research with an abundance of research monkeys — Chinese researchers face less red tape than those in the West when it comes to research on primates — could be powerful. “If you want to test vaccines or antivirals, you need a non-human primate model,” says Lina.
But Ebright is not convinced of the need for more than one BSL-4 lab in mainland China. He suspects that the expansion there is a reaction to the networks in the United States and Europe, which he says are also unwarranted. He adds that governments will assume that such excess capacity is for the potential development of bioweapons.
“These facilities are inherently dual use,” he says. The prospect of ramping up opportunities to inject monkeys with pathogens also worries, rather than excites, him: “They can run, they can scratch, they can bite.”
Trevan says China’s investment in a BSL-4 lab may, above all, be a way to prove to the world that the nation is competitive. “It is a big status symbol in biology,” he says, “whether it’s a need or not.”
Interestingly, in addition to Wuhan, the Chinese government planned to open a new BSL-4 lab in Kunming, with an eye to testing vaccines on primates. As you might recall, Kunming is not only the capital of Yunnan, but it is also where Shi Zhengli found the strains Rs3367 and RsSHC014 in nearby caves. By the way, primate testing was mentioned by Baric and Shi Zhengli as possible next steps for the development of preventive vaccines against potential future outbreaks of coronaviruses in their famous 2015 paper:
However, further testing in nonhuman primates is required to translate these finding into pathogenic potential in humans. Importantly, the failure of available therapeutics defines a critical need for further study and for the development of treatments. With this knowledge, surveillance programs, diagnostic reagents and effective treatments can be produced that are protective against the emergence of group 2b–specific CoVs, such as SHC014, and these can be applied to other CoV branches that maintain similarly heterogeneous pools.
Maybe by 2019 the creation and testing of potential vaccines against various SARS-like coronaviruses was already in full swing.
- admin
- Site Admin
- Posts: 40066
- Joined: Thu Aug 01, 2013 5:21 am
Re: U.S. government gave $3.7 million grant to Wuhan lab at
Part 3 of 3
Beware of Lab
Let’s now take a look at the lab leak hypothesis. But first, I will provide some historical context, including previous confirmed lab leaks, as many of those happened before. First and foremost, lab leaks of the first SARS-CoV: initially, in the summer of 2003 in Singapore, then in December 2003 in Taiwan, and in the spring of 2004 twice in Beijing.
There were close calls in Europe and the USA, although thankfully no infections occurred there. For example, a French lab once lost vials with SARS, and an American BSL-4 laboratory in Texas, lost a vial containing Guanarito (Venezuelan hemorrhagic fever virus):
History knows other, much larger-scale leaks. For example, the “resurrection” of the H1N1 flu virus in 1977, which had previously been considered extinct. Yes, this is the virus of the famous “Spanish flu”:
It seems that the creation of temperature-sensitive viral mutants to develop potentially attenuated vaccines was widespread at the end of the twentieth century. If you remember, in 1990, Ralph Baric himself also experimented with the creation of temperature-sensitive coronavirus strains.
Could something like this have caused the Covid-19 pandemic? Several options are possible — from a leak during development of a potential vaccine to fundamental research on laboratory recombination of the bat and pangolin viruses. Some particularly ambitious researcher could even decide to combine the two “fashionable research themes” — adding a furin site and transplanting RBM from a strain of one species (pangolin) to another (bats), so that later, confirming the increased virulence of the new chimeric virus, they can wax poetic about the dangers of the same recombination happening in Yunnan caves or wet markets. And if such a researcher could even pre-emptively develop a vaccine against this and other potential chimeras, all sorts of accolades could await.
Am I then saying this is what happened? Of course not, I do not claim to know what happened. Today, there is no evidence of this. For now, there is just a series of strange coincidences — for example, that the outbreak of the Yunnan coronavirus occurred thousands of kilometers from Yunnan in a wet market closest to the Wuhan Institute of Virology. Or maybe not at the wet market, as 3 of the first 4 patients had no ties to the market. Plus, there are coincidences in the structural features of the CoV2 genome, which resemble manipulations that virologists have repeatedly carried out in the lab. But coincidence is not proof.
Moreover, coincidences happen, and CoV2 could obviously have arisen naturally. It is not yet clear exactly how — for this, the bat and pangolin strains must have met in the same cell of some animal in Wuhan, since the outbreak occurred there (otherwise we would have seen other outbreaks along the path that animal would have taken to get to Wuhan). Given that bats were not sold in the Wuhan market, and generally hibernate at this time of the year, and that no other carriers of ancestral strains have yet been identified, the exact scenario of natural emergence remains a mystery.
On the opposite side of the balance, giving credence to the lab hypothesis, there are reports that in 2018, American experts were quite alarmed after their visit to the Wuhan Institute of Virology and conversation with Shi Zhengli. Their “lab tour” resulted in two diplomatic dispatches to Washington in which they noted a number of safety weaknesses:
The Chinese researchers at WIV were receiving assistance from the Galveston National Laboratory at the University of Texas Medical Branch and other U.S. organizations, but the Chinese requested additional help. The cables argued that the United States should give the Wuhan lab further support, mainly because its research on bat coronaviruses was important but also dangerous.
It is somewhat ironic the Wuhan lab received guidance from the Texas laboratory in Galveston, which at one time had itself lost a vial with a Guanarito virus: Wuhan specialists were trained at Galveston, which was even reported in the Wuhan Institute’s own newsletter (though, that publication has been deleted from the WIV website, but it is still available at the Wayback Machine):

A training session in Galveston National Laboratory. Credit: Courtesy of GNL/UTMB
A couple of final touches to the family portrait of laboratory leaks: in November 2019, an outbreak of brucellosis (a bacterial infection) occurred in two research centers in Lanzhou, China, infecting over 100 researchers who worked there. American labs have also not been immune to outbreaks, although not on the same scale:
Possible Hallmarks of Lab Origin?
Let us now turn our attention back to the virus itself. Does it have any obvious signs of lab manipulation? First, a few words about what “obvious” means. Any mutation can arise naturally, and even if the amino acid insert that had created the furin site in CoV2 was not “PRRA” but “MADEINWVHANPRRA”, there would still be a non-zero chance that it arose by accident. But for us, and for any court, I think this would be enough to prove lab origin beyond a reasonable doubt.
The main problem with such evidence is that even in a lab-made virus it simply may not exist. Basically, a good genetic engineer can create a synthetic virus that would be indistinguishable from a natural one. Moreover, often researchers deliberately introduce some synonymous mutations into their designs so that later they can discern their strain from natural ones. But if the creators choose not to reveal these markers, it is impossible to distinguish them from natural mutations.
But sometimes traces of manipulation may remain, especially if the creators do not try to hide them. First of all, I am talking about the spots in virus genome where its DNA is cut (recall that RNA virus manipulations are carried out in complementary DNA constructs). This occurs when virus creators need cut out a segment, or stitch together new segments. After all, DNA cannot be cut in arbitrary places (CRISPR aside), but only where its nucleotide sequence (usually 4–6 “letters”) forms a sequence recognized by some restriction enzyme, that is, an enzyme that can cut a nucleotide chain. However, such an analysis is complicated by the fact that there are hundreds of different types of restriction enzymes used in genetic engineering. But let’s try it anyways.
As a baseline, here is an example of the work of the Baric group from 2008, where they took Bat-SCoV and replaced its RBD by an RBD from human SARS. Here’s how they describe the creation of their chimera:
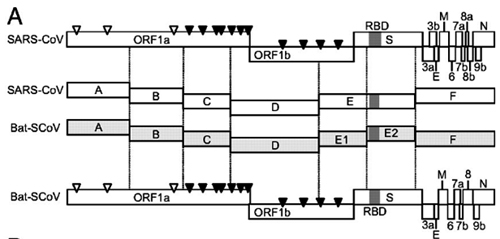
Schematic representation of SARS-CoV and Bat-SCoV variants.
(A) Schematic representation of SARS-CoV and Bat-SCoV (GenBank accession no. FJ211859) genomes and reverse genetics system. (Top) Arrowheads indicate nsp processing sites within the ORF1ab polyprotein (open arrowheads, papain-like proteinase mediated; filled arrowheads, nsp5 [3C-like proteinase] mediated). Immediately below are the fragments used in the reverse genetics system, labeled A through F. The fragments synthesized to generate Bat-SCoV exactly recapitulate the fragment junctions of SARS-CoV with the exception that the Bat-SCoV has 2 fragments, Bat-E1 and Bat-E2, which correspond to the SARS-E fragment.
As you can see, the Baric group first created a synthetic clone of Bat-SCoV in the same pattern as they used for their synthetic clone of SARS-CoV. That is, for the bat clone, they used the same 6 segments with the same restriction enzyme sites that they had previously used for SARS-CoV, which allowed them to swap virus segments between different strains like Lego pieces. Here is their detailed description:
All these three-letter abbreviations (BglI, AflII, NotI, etc.) in the sentence highlighted above are different types of restriction enzymes. Let’s see if there are any differences in the restriction enzyme sites in the spike protein sequence of the chimera compared to the genome of the original SARS-CoV:
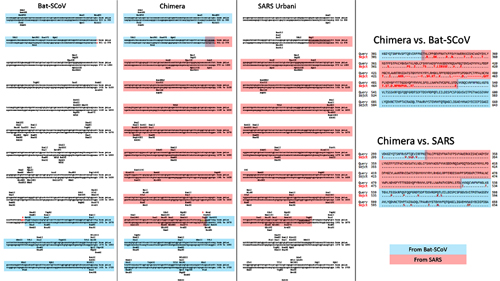
As can be seen, the restriction enzyme sites of the chimera are almost identical to those in the original sequences in Bat-SCoV or SARS from where they were taken. The only differences are noticeable at the “stitching” sites of the inserted SARS piece. Here, for example, is the left (5’-) edge of the insert:
Here Bat-SCoV and SARS turned out to have a common identical region of nucleotides (the intersection of cyan and pink regions), and there are no new restriction enzyme sites at the stitching site of the two sequences, on the contrary, the SspI site from SARS disappeared. And here is the right (3’-) edge of the insert:
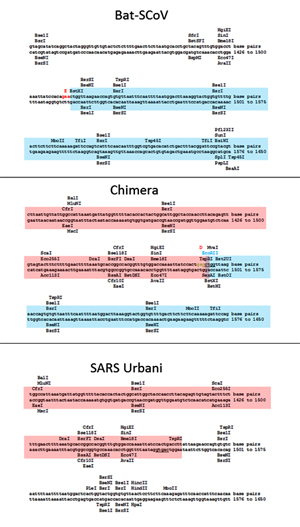
Here, on the contrary, all the original restriction enzyme sites remain at the site of ligation, and even new ones appear, for example, EcoRII. Had I not known that the chimeric genome is the result of lab manipulations, could I deduce this by looking at these 3 sequences? Not really, and even if some suspicion did creep in, it would certainly not be beyond a reasonable doubt. Perhaps it would be obvious to specialists in genetic engineering by some other signs, and, if so, I hope they speak up.
But in any case, let’s compare the RaTG13spike protein to CoV2 and pangolin-2019. Just in case something does jump out.
This is what the RBD (highlighted in light green) and RBM (yellow) look like for all three:
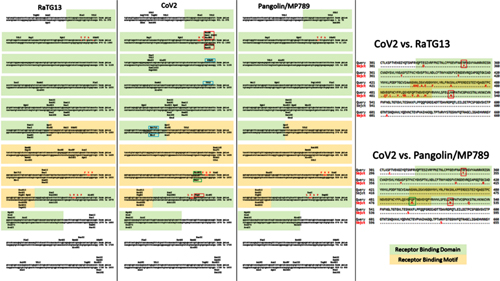
So, anything interesting? Well, I noticed some new restriction enzyme sites in CoV2 marked by red rectangles — they coincide with unique mutations in the amino acid sequence (also marked by red rectangles in the amino acid sequences on the far right). Just in case, I highlighted several other new sites: blue rectangles, and a green rectangle located in the region of the only amino acid that differs between RBMs of CoV2 and pangolin-2019.
Let’s now compare the stretch around the PRRA insert that created the furin site in CoV2 among those three strains:
Here, too, several new restriction sites have appeared (highlighted in blue) on both sides of the new insert. Could they have been used to create a furin site? Theoretically, yes. Alternatively, the insertion could have been made via existing sites or even using the “seamless” ligation method — i.e. by creation of segments with new restriction sites which disappear after the complementary ends are joined. You might remember that the Baric group have applied this technology in 2002 to create a synthetic clone of murine coronavirus:
In 2003 they have used this approach again for a synthetic clone of SARS-CoV:
Today, genetic manipulation techniques are so advanced and have become so routine that the October 2019 Beijing paper which had inserted a new furin site into the chicken coronavirus, only devoted a couple of sentences to their methodology:
The pace of progress in genetic engineering is astounding. Here is a description of the above Seamless Assembly kit:
Up to 4 DNA fragments can be joined in a desired orientation in about half an hour, without having to deal with restriction enzymes or ligation. Once you’re done, quickly “upload” your creation into E. coli to propagate the resulting design. Easy-peasy!
In summary, the restriction enzyme site analysis did not yield anything conclusive. It did, however, point out that not only CoV2 is quite unique, but so is RaTG13, and we should continue digging into the origins of both.
Codon Preferences
For these purposes, I decided to take a look at codon usage bias to check which strains look like CoV2 and RaTG13 the most. It is known that viruses tend to adapt their codon signature to the preferences of their hosts, so I expected to see RaTG13 exhibit a similar pattern to other bat viruses, and also hoped to see a difference from pangolin strains.
SARS-CoV, for example, is very similar to Rs3367 and RsSCH014, as one might expect:
Among themselves, by the way, SARS, MERS and CoV2 do differ:
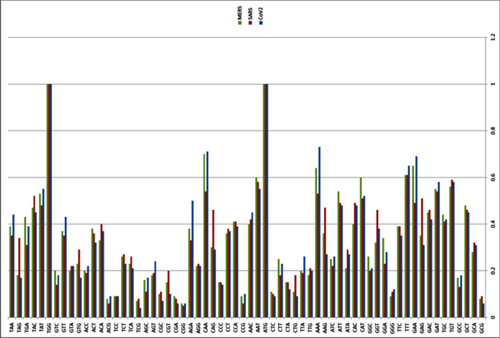
RaTG13 is similar to CoV2, which is also to be expected:
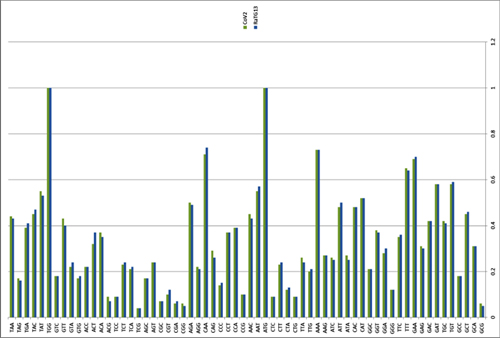
But RaTG13 is actually not that close to the pangolin strains, and the pangolin strains are not exactly identical to each other:
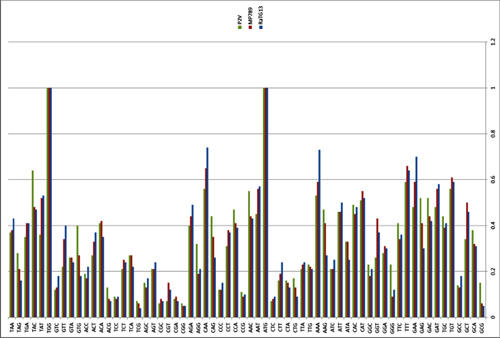
RaTG13 also differs from ZXC21 and ZC45:
Looking at Yunnan strains, RaTG13 is quite distant from Rs3367 and RsSCH014, and closer to LYRa11, but also with noticeable differences:
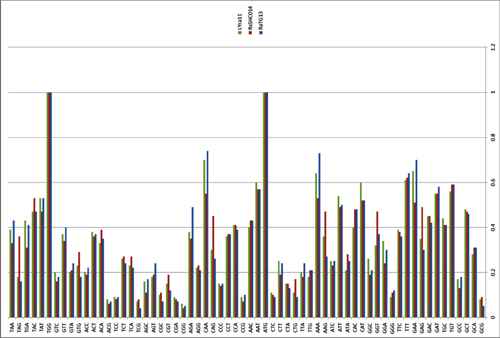
In general, as before, RaTG13 and CoV2 stand out in a class of their own. I was also intrigued by the AAA codon — they use it much more often than their fellow strains:
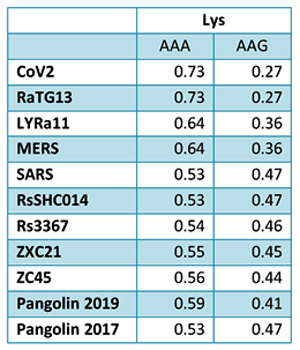
This is probably just another coincidence, but a similar proportion between AAA and AAG is observed in E. coli. Can the cDNA codon signature change if it is being cultivated for a long time in cell culture? Maybe, but I haven’t yet dug into this topic very deeply.
[UPDATED] I also decided to check codon usage patterns between RaTG13 and other Ra strains collected from the same abandoned mineshaft in Mojiang where in 2013 Shi Zhengli’s group found strain RaBtCoV/4991 (KP876546) that shares an identical 370-bp RdRp segment with RaTG13. Unfortunately, only 816-bp segments of the RdRp gene were available for the other Ra strains (RaBtCoV/3750 and RaBtCoV/4307–2), so I extracted the corresponding 816-bp segment from RaTG13 for the purposes of codon usage comparison. RaTG13 again differed substantially, while the other two strains clustered together:
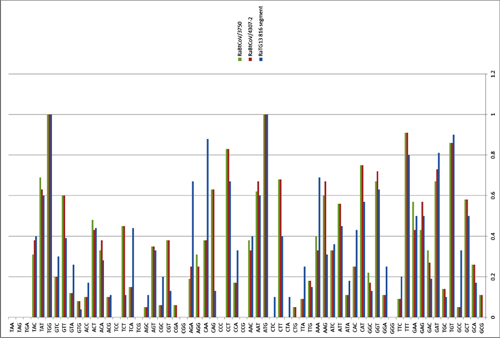
So codon analysis also did not reveal any obvious signs of lab origins, but once again confirmed the uniqueness of CoV2 and RaTG13. What does this leave us with? So far, just a number of oddities, which, as scientists like to say, taken together, do not allow us to reject the lab origin hypothesis of CoV2.
The Nature Paper vs. the Lab-Made Hypothesis
But didn’t that Nature article refute the lab-made hypothesis? No, not really. There is no irrefutable evidence against it in the paper, just a loud “we don’t believe so” based on a shaky foundation. Judge for yourself — here are the authors’ key arguments in support of their conclusions:
In the original paper, the quoted sentences are just below the diagram showing identical RBMs between CoV2 and pangolin-2019. So I am puzzled as to what “computational analysis” has to do with anything. Obviously, the most likely scenario for the lab-made hypothesis is the transfer of RBM from one strain to another — which virologists have done many times before. Therefore, the author’s chain of arguments does not make sense: “computer says binding is not ideal, thus CoV2 must be the result of natural selection. Ergo, this is strong evidence that CoV2 is not lab-made.” Wait, just because CoV2 differs from some “optimal” virus, doesn’t mean it could not have been created in a lab. Not the lab trying to create “optimal” bioweapons, but a lab creating chimeras of naturally found strains, say, in bats and pangolins.
The authors continue to surprise:
Again, the same questionable logic dressed in categorical adjectives: “genetic analysis irrefutably proves that CoV2 was not created on the basis of previously known strains!” Well thanks, Captain Obvious. But why couldn’t potential creators of CoV2 make a cDNA backbone from unpublished strains related to or even derived from RaTG13? Then they could easily insert the pangolin RBM into it, as well as add a furin site (or maybe the cDNA backbone already had one). Virologists have been doing things like this for 20 years, and modern genetic engineering tools make such manipulations accessible even to a grad student.
As for the chances of the furin site arising in cell culture, the authors also express strange ideas:
First off, the authors themselves cite previous works where the furin site arose in vitro as viruses were cultured in cells. And second, what do they mean, a strain with high genetic similarity has not been described — what about RaTG13? If it had its RBM replaced by one from the pangolin strain, and then the chimeric strain was cultured in vitro, then the furin site could well have arisen in this matter. Additionally, the new strain could thus acquire other mutations that distinguish CoV2 from RaTG13 and pangolin-2019.
But in terms of the potential lab-based origin of the furin site, I am more inclined to hypothesize a specific insertion — as in the Beijing paper from October 2019 with chicken coronavirus. After that, the synthetic strain could have acquired new mutations by subsequent culturing in vitro or in vivo — like the MA15 murine strain in 2007, for example. Or maybe even using the same mouse model with humanized lung tissues and immune system that was created at UNC by Baric’s and other groups in 2018, in which they reported testing several viruses including MERS:
Finally, even if CoV2 is the product of selection rather than intelligent design, that does not rule out a lab leak either — selection can happen in the lab just as well, both natural and artificial kinds. Different strains can recombine in research animals or in vitro by design or by chance.
On the 4% Genome Difference between RaTG13 and Cov2
Some critics of the lab-made hypothesis claim that the observed ~4% genetic difference between RaTG13 and CoV2 is too high to have possibly occurred in a lab if RaTG13 itself was used as a backbone. Observed mutation rates for RNA viruses vary widely — from 10⁻⁶ to 10⁻⁴ nucleotides per replication in vitro, and in humans CoV2 seems to mutate at a rate of 25 mutations per year. Thus, the logic goes, it would take years, if not decades, for two strains to diverge by 4%. While that is a valid point, there are several issues with that line of reasoning.
First, in vitro mutation speeds (i.e. per unit of time) are much higher, as you can passage cells much more often than infect new animals. As SARS and MERS in vitro experiments showed, significant mutations might be observed after only a few passages. For example, the 2004 paper reported that only after 600 passages there already was a 2.1% difference in the genomic sequences of spike proteins between the original strain and its progeny:
Moreover, in the presence of some antiviral compounds, such as nucleoside analogs (e.g. ribavirin or remdesivir), mutation rates in RNA viruses can increase even further:
So if ancestral CoV2 was being lab-tested to assess how its mutagenesis might affect the efficacy of potential vaccines or antiviral drugs, it could have accumulated mutations at a much higher rate.
But possibly, the biggest problem with the 4% difference argument is that it relies on RaTG13 being exactly what WIV says it is. If we are to seriously consider the lab leak hypothesis, we must concede that it does not make sense to blindly trust the data released by the very lab suspected of the leak. If the leak did occur, as is the premise of the lab hypothesis, then the description of what RaTG13 is could be furthering the goal of covering up the leak.
Again, I am not claiming with certainty that is what is happening here. All I am saying is that this is what could have happened, and we need a lot more evidence before we can reach a definitive conclusion. One thing that could help rule out tampering with RaTG13 is having independent labs sequence the 2013 Yunnan samples that She Zhengli extracted RaTG13 from. WIV must still have them if they re-sequenced RaTG13 in 2020.
Shi Zhengli-2020
As I was writing this post, a fresh paper co-authored by Shi Zhengli came out, in which the authors tested a peptide which they have been studying for some time before against CoV2. That peptide was meant to be a pan-coronavirus inhibitor, and its designed mode of action was to block the fusion of a spike protein with a cell membrane. The authors, of course, mention the new furin site of CoV2, and suggest that it may play an important role in the much more efficient penetration of CoV2 into the cell:
In this context, I wonder whether the authors have previously conducted experiments on how adding a furin site can alter the effectiveness of their peptide (or other drugs or vaccines) against a given coronavirus.
Not to be outdone, Ralph Baric also joined the race to find drugs against CoV2. As I understand, he and co-authors took data on the effectiveness of their nucleoside analogue (β-D-N4-hydroxycytidine, NHC) against SARS-CoV and MERS that they already had, added some in vitro data on CoV2, and sent off the paper to print. Nucleoside analogues (such as the famous remdesivir) are a fundamentally different approach than Shi Zhengli et al. Here, the authors try to prevent viral replication by giving “defective” letters of the genetic alphabet to virus’ copying machine, while Shi Zhengli and coauthors try to prevent the virus from entering the cell altogether. Theoretically, these approaches could be combined.
This is the End, Beautiful Friend
If you made it here by reading rather than scrolling, mad props to you. Hey, even if you scrolled, that’s cool too, and I apologize for the verbosity. I just didn’t anticipate that the rabbit hole would turn out to be a whole underground cave system. I hope that you found this deep dive into the world of virology interesting and enjoyed the exploration of the lab-made CoV2 hypothesis. In my opinion, the data I have presented, taken together, do not allow us to reject this possibility.
Let me be clear: this does NOT prove that CoV2 was synthesized in the laboratory. Yes, as we have seen above, from a technical standpoint, it would not be difficult for a modern virologist to create such a strain. But there is no direct evidence that anyone did this, and strange coincidences cannot pass for circumstantial evidence. On balance, the current chances against this are still higher than for the natural origins of CoV2. Moreover, even if CoV2 was indeed an unfortunate lab leak, the scientists themselves are not to blame, as they were working within the established international laws and guidelines on such research. Now, those who might be trying to cover up that leak, that’s a different story.
The opposite point is worth repeating too: the inverse hypothesis about the exclusively natural origin of the virus does not yet have strong evidence either. Until intermediate ancestors between RaTG13, pangolin-2019 and CoV2 are found, in whom we could trace the mosaic recombination that we observe in CoV2, the question of its origins remains open.
In closing, there is no one better to quote on this matter than Ralph Baric himself:
So there you have it. It remains possible that the mysterious virus host was a lab:

Bad pun? Sorry, last one.
How I Learned to Hate the GOF
I hope this post is not used to prematurely assign blame or propagate one-sided theories. What I do hope it highlights is the scale of dangerous gain-of-function research that has been and is going on in virology. The Covid-19 pandemic really exposed its huge risks in the face of few benefits: GOF research hasn’t protected us from this outbreak, hasn’t provided us with any effective treatments or vaccines in time to save hundreds of thousands of lives lost to CoV2, and if there is even a 0.1% chance GOF research caused the whole thing, that chance is too high.
Beware of Lab
Let’s now take a look at the lab leak hypothesis. But first, I will provide some historical context, including previous confirmed lab leaks, as many of those happened before. First and foremost, lab leaks of the first SARS-CoV: initially, in the summer of 2003 in Singapore, then in December 2003 in Taiwan, and in the spring of 2004 twice in Beijing.
There were close calls in Europe and the USA, although thankfully no infections occurred there. For example, a French lab once lost vials with SARS, and an American BSL-4 laboratory in Texas, lost a vial containing Guanarito (Venezuelan hemorrhagic fever virus):
Only one scientist worked with the virus, and Reyes said the lab suspects that scientist accidentally threw the vial away in November.
…
Galveston biolab requires the most stringent safety measures because it studies biosafetly level BSL-4 materials, or dangerous infectious diseases that have no vaccines or cures. BSL-4 materials include Guanarito, Ebola and smallpox.
History knows other, much larger-scale leaks. For example, the “resurrection” of the H1N1 flu virus in 1977, which had previously been considered extinct. Yes, this is the virus of the famous “Spanish flu”:
Human influenza H1N1 viruses appeared with the 1918 pandemic, and persisted, slowing accumulating small changes in its genome (with a major change in 1947), until the H2N2 “Asian” flu appeared in 1957, causing a worldwide pandemic. H1N1 influenza virus then apparently became extinct, and was not isolated for 20 years. In 1969 the “Hong Kong” H3N2 virus replaced the H2N2 virus, and is still circulating.
In September 1977 an H1N1 influenza virus was isolated from human infections in the Far East region of the Soviet Union, and in early 1978 the Chinese reported they had isolated H1N1 virus in May of 1977 in northeast China adjacent to the Soviet outbreak. Using the early genetic tools available at the time, the 1977 H1N1 virus was found to be closely related to H1N1 human influenza viruses circulating in 1949–1950, but not to those circulating earlier or later.
…
Only since 2009–2010 did major papers begin to state directly the 1977 emergence of H1N1 influenza was a laboratory related release: “The most famous case of a released laboratory strain is the re-emergent H1N1 influenza A virus which was first observed in China in May of 1977 and in Russia shortly thereafter.”
…
The speculation that the 1977 release may have been related to H1N1 vaccine research is supported by the observation that in the initial outbreaks in China, nine of the ten viral isolates expressed “temperature sensitivity” (Kung 1978). Temperature sensitivity normally an uncommon trait, but one that was in the 1970s (and still is) a fundamental trait for making live attenuated influenza vaccines. Temperature sensitivity generally occurs only after a series of substantial laboratory manipulations and selections.
Interestingly, further investigation indicated the circulating strains in 1977–78 were often comprised of mixed temperature-sensitive and normal components, and that temperature sensitivity apparently disappeared from the post-1978 H1N1 lineage rapidly. Escape of a mid-protocol population of H1N1 virus undergoing laboratory selection for temperature sensitive mutants would provide such a mixed population. In 1976–77 laboratory personnel in their late teens or early 20s would not have been exposed to pre-1957 H1N1 influenza viruses, and been susceptible to laboratory infections. The low severity of the 1977 pandemic might be in part due to the temperature sensitivity of the virus, a trait that limits virus replication in pulmonary tissues.
It seems that the creation of temperature-sensitive viral mutants to develop potentially attenuated vaccines was widespread at the end of the twentieth century. If you remember, in 1990, Ralph Baric himself also experimented with the creation of temperature-sensitive coronavirus strains.
Could something like this have caused the Covid-19 pandemic? Several options are possible — from a leak during development of a potential vaccine to fundamental research on laboratory recombination of the bat and pangolin viruses. Some particularly ambitious researcher could even decide to combine the two “fashionable research themes” — adding a furin site and transplanting RBM from a strain of one species (pangolin) to another (bats), so that later, confirming the increased virulence of the new chimeric virus, they can wax poetic about the dangers of the same recombination happening in Yunnan caves or wet markets. And if such a researcher could even pre-emptively develop a vaccine against this and other potential chimeras, all sorts of accolades could await.
Am I then saying this is what happened? Of course not, I do not claim to know what happened. Today, there is no evidence of this. For now, there is just a series of strange coincidences — for example, that the outbreak of the Yunnan coronavirus occurred thousands of kilometers from Yunnan in a wet market closest to the Wuhan Institute of Virology. Or maybe not at the wet market, as 3 of the first 4 patients had no ties to the market. Plus, there are coincidences in the structural features of the CoV2 genome, which resemble manipulations that virologists have repeatedly carried out in the lab. But coincidence is not proof.
Moreover, coincidences happen, and CoV2 could obviously have arisen naturally. It is not yet clear exactly how — for this, the bat and pangolin strains must have met in the same cell of some animal in Wuhan, since the outbreak occurred there (otherwise we would have seen other outbreaks along the path that animal would have taken to get to Wuhan). Given that bats were not sold in the Wuhan market, and generally hibernate at this time of the year, and that no other carriers of ancestral strains have yet been identified, the exact scenario of natural emergence remains a mystery.
On the opposite side of the balance, giving credence to the lab hypothesis, there are reports that in 2018, American experts were quite alarmed after their visit to the Wuhan Institute of Virology and conversation with Shi Zhengli. Their “lab tour” resulted in two diplomatic dispatches to Washington in which they noted a number of safety weaknesses:
Sources familiar with the cables said they were meant to sound an alarm about the grave safety concerns at the WIV lab, especially regarding its work with bat coronaviruses. The embassy officials were calling for more U.S. attention to this lab and more support for it, to help it fix its problems.
…
“During interactions with scientists at the WIV laboratory, they noted the new lab has a serious shortage of appropriately trained technicians and investigators needed to safely operate this high-containment laboratory,” states the Jan. 19, 2018, cable, which was drafted by two officials from the embassy’s environment, science and health sections who met with the WIV scientists. (The State Department declined to comment on this and other details of the story.)
The Chinese researchers at WIV were receiving assistance from the Galveston National Laboratory at the University of Texas Medical Branch and other U.S. organizations, but the Chinese requested additional help. The cables argued that the United States should give the Wuhan lab further support, mainly because its research on bat coronaviruses was important but also dangerous.
It is somewhat ironic the Wuhan lab received guidance from the Texas laboratory in Galveston, which at one time had itself lost a vial with a Guanarito virus: Wuhan specialists were trained at Galveston, which was even reported in the Wuhan Institute’s own newsletter (though, that publication has been deleted from the WIV website, but it is still available at the Wayback Machine):

A training session in Galveston National Laboratory. Credit: Courtesy of GNL/UTMB
A couple of final touches to the family portrait of laboratory leaks: in November 2019, an outbreak of brucellosis (a bacterial infection) occurred in two research centers in Lanzhou, China, infecting over 100 researchers who worked there. American labs have also not been immune to outbreaks, although not on the same scale:
Possible Hallmarks of Lab Origin?
Let us now turn our attention back to the virus itself. Does it have any obvious signs of lab manipulation? First, a few words about what “obvious” means. Any mutation can arise naturally, and even if the amino acid insert that had created the furin site in CoV2 was not “PRRA” but “MADEINWVHANPRRA”, there would still be a non-zero chance that it arose by accident. But for us, and for any court, I think this would be enough to prove lab origin beyond a reasonable doubt.
The main problem with such evidence is that even in a lab-made virus it simply may not exist. Basically, a good genetic engineer can create a synthetic virus that would be indistinguishable from a natural one. Moreover, often researchers deliberately introduce some synonymous mutations into their designs so that later they can discern their strain from natural ones. But if the creators choose not to reveal these markers, it is impossible to distinguish them from natural mutations.
But sometimes traces of manipulation may remain, especially if the creators do not try to hide them. First of all, I am talking about the spots in virus genome where its DNA is cut (recall that RNA virus manipulations are carried out in complementary DNA constructs). This occurs when virus creators need cut out a segment, or stitch together new segments. After all, DNA cannot be cut in arbitrary places (CRISPR aside), but only where its nucleotide sequence (usually 4–6 “letters”) forms a sequence recognized by some restriction enzyme, that is, an enzyme that can cut a nucleotide chain. However, such an analysis is complicated by the fact that there are hundreds of different types of restriction enzymes used in genetic engineering. But let’s try it anyways.
As a baseline, here is an example of the work of the Baric group from 2008, where they took Bat-SCoV and replaced its RBD by an RBD from human SARS. Here’s how they describe the creation of their chimera:

Schematic representation of SARS-CoV and Bat-SCoV variants.
(A) Schematic representation of SARS-CoV and Bat-SCoV (GenBank accession no. FJ211859) genomes and reverse genetics system. (Top) Arrowheads indicate nsp processing sites within the ORF1ab polyprotein (open arrowheads, papain-like proteinase mediated; filled arrowheads, nsp5 [3C-like proteinase] mediated). Immediately below are the fragments used in the reverse genetics system, labeled A through F. The fragments synthesized to generate Bat-SCoV exactly recapitulate the fragment junctions of SARS-CoV with the exception that the Bat-SCoV has 2 fragments, Bat-E1 and Bat-E2, which correspond to the SARS-E fragment.
As you can see, the Baric group first created a synthetic clone of Bat-SCoV in the same pattern as they used for their synthetic clone of SARS-CoV. That is, for the bat clone, they used the same 6 segments with the same restriction enzyme sites that they had previously used for SARS-CoV, which allowed them to swap virus segments between different strains like Lego pieces. Here is their detailed description:
Viruses containing PCR-generated insertions within the viral coding sequence were produced by using the SARS-CoV assembly strategy (24, 33, 53) with the following modifications. Briefly, for Bat-F virus, full-length cDNA was constructed by ligating restriction products from SARS-CoV fragments A–E and Bat-SCoV fragment F, which required a BglI-NotI digestion. For Bat-SCoV and Bat-SRBD, Bat-SRBM, and Bat-Hinge, plasmids containing the 7 cDNA fragments of the Bat-SCoV genome were digested by using BglI for Bat-A, Bat-B, Bat-C, and Bat-D, BglI and AflII for Bat-E1 and Bat-E2, and BglI and NotI for Bat-F. Digested, gel-purified fragments were simultaneously ligated together. Transcription was driven by using a T7 mMessage mMachine kit (Ambion), and RNA was electroporated into Vero cells (24, 53).
All these three-letter abbreviations (BglI, AflII, NotI, etc.) in the sentence highlighted above are different types of restriction enzymes. Let’s see if there are any differences in the restriction enzyme sites in the spike protein sequence of the chimera compared to the genome of the original SARS-CoV:

As can be seen, the restriction enzyme sites of the chimera are almost identical to those in the original sequences in Bat-SCoV or SARS from where they were taken. The only differences are noticeable at the “stitching” sites of the inserted SARS piece. Here, for example, is the left (5’-) edge of the insert:

Here Bat-SCoV and SARS turned out to have a common identical region of nucleotides (the intersection of cyan and pink regions), and there are no new restriction enzyme sites at the stitching site of the two sequences, on the contrary, the SspI site from SARS disappeared. And here is the right (3’-) edge of the insert:

Here, on the contrary, all the original restriction enzyme sites remain at the site of ligation, and even new ones appear, for example, EcoRII. Had I not known that the chimeric genome is the result of lab manipulations, could I deduce this by looking at these 3 sequences? Not really, and even if some suspicion did creep in, it would certainly not be beyond a reasonable doubt. Perhaps it would be obvious to specialists in genetic engineering by some other signs, and, if so, I hope they speak up.
But in any case, let’s compare the RaTG13spike protein to CoV2 and pangolin-2019. Just in case something does jump out.
This is what the RBD (highlighted in light green) and RBM (yellow) look like for all three:

So, anything interesting? Well, I noticed some new restriction enzyme sites in CoV2 marked by red rectangles — they coincide with unique mutations in the amino acid sequence (also marked by red rectangles in the amino acid sequences on the far right). Just in case, I highlighted several other new sites: blue rectangles, and a green rectangle located in the region of the only amino acid that differs between RBMs of CoV2 and pangolin-2019.
Let’s now compare the stretch around the PRRA insert that created the furin site in CoV2 among those three strains:

Here, too, several new restriction sites have appeared (highlighted in blue) on both sides of the new insert. Could they have been used to create a furin site? Theoretically, yes. Alternatively, the insertion could have been made via existing sites or even using the “seamless” ligation method — i.e. by creation of segments with new restriction sites which disappear after the complementary ends are joined. You might remember that the Baric group have applied this technology in 2002 to create a synthetic clone of murine coronavirus:
The interconnecting restriction site junctions that are located at the ends of each cDNA are systematically removed during the assembly of the complete full-length cDNA product, allowing reassembly without the introduction of nucleotide changes.
In 2003 they have used this approach again for a synthetic clone of SARS-CoV:
To rapidly assemble consensus clones, we used class IIS restriction endonucleases that cut at asymmetric sites and leave asymmetric ends. These enzymes generate strand-specific unique overhangs that allow the seamless ligation of two cDNAs with the concomitant loss of the restriction site.
Today, genetic manipulation techniques are so advanced and have become so routine that the October 2019 Beijing paper which had inserted a new furin site into the chicken coronavirus, only devoted a couple of sentences to their methodology:
2.2. Generation of Recombinant Virus
Recombinant rYN-S2/RRKR virus containing an S protein with the furin-S2′ site was generated by vaccinia recombination, as described previously [20,28]. Briefly, plasmid with the furin-S2′ site was generated using the Seamless Assembly kit (Invitrogen, Carlsbad, CA, USA) and transfected into CV-1 cells infected by vaccinia virus containing the genome of YN-ΔS-GPT. Furin-S2’ site was introduced into the YN cDNA by homologous recombination using the transient dominant selection system [25].
The pace of progress in genetic engineering is astounding. Here is a description of the above Seamless Assembly kit:
The GeneArt® Seamless Cloning and Assembly Kit enables the simultaneous and directional cloning of 1 to 4 PCR fragments, consisting of any sequence, into any linearized vector, in a single 30-minute room temperature reaction. The kit contains everything required for the assembly of DNA fragments, and their transformation into E. coli for selection and growth of recombinant vectors.
• Speed and Ease — Clone up to 4 DNA fragments, with sequence of your choice, simultaneously in a single vector (up to 13 Kb); no restriction digestion, ligation or recombination sites required
• Precision and Efficiency — Designed to let you clone what you want, where you want, in the orientation you want, and achieve up to 90% correct clones with no extra sequences left behind
• Vector Flexibility — Use our linear vector or a vector of your choice
• Free Tools — Design DNA oligos and more with our free web-based interface that walks you step-by-step through your project
• Diverse Applications — Streamline many synthetic biology and molecular biology techniques through the rapid combination, addition, deletion, or exchange of DNA segments
Up to 4 DNA fragments can be joined in a desired orientation in about half an hour, without having to deal with restriction enzymes or ligation. Once you’re done, quickly “upload” your creation into E. coli to propagate the resulting design. Easy-peasy!
In summary, the restriction enzyme site analysis did not yield anything conclusive. It did, however, point out that not only CoV2 is quite unique, but so is RaTG13, and we should continue digging into the origins of both.
Codon Preferences
For these purposes, I decided to take a look at codon usage bias to check which strains look like CoV2 and RaTG13 the most. It is known that viruses tend to adapt their codon signature to the preferences of their hosts, so I expected to see RaTG13 exhibit a similar pattern to other bat viruses, and also hoped to see a difference from pangolin strains.
SARS-CoV, for example, is very similar to Rs3367 and RsSCH014, as one might expect:

Among themselves, by the way, SARS, MERS and CoV2 do differ:

RaTG13 is similar to CoV2, which is also to be expected:

But RaTG13 is actually not that close to the pangolin strains, and the pangolin strains are not exactly identical to each other:

RaTG13 also differs from ZXC21 and ZC45:

Looking at Yunnan strains, RaTG13 is quite distant from Rs3367 and RsSCH014, and closer to LYRa11, but also with noticeable differences:

In general, as before, RaTG13 and CoV2 stand out in a class of their own. I was also intrigued by the AAA codon — they use it much more often than their fellow strains:

This is probably just another coincidence, but a similar proportion between AAA and AAG is observed in E. coli. Can the cDNA codon signature change if it is being cultivated for a long time in cell culture? Maybe, but I haven’t yet dug into this topic very deeply.
[UPDATED] I also decided to check codon usage patterns between RaTG13 and other Ra strains collected from the same abandoned mineshaft in Mojiang where in 2013 Shi Zhengli’s group found strain RaBtCoV/4991 (KP876546) that shares an identical 370-bp RdRp segment with RaTG13. Unfortunately, only 816-bp segments of the RdRp gene were available for the other Ra strains (RaBtCoV/3750 and RaBtCoV/4307–2), so I extracted the corresponding 816-bp segment from RaTG13 for the purposes of codon usage comparison. RaTG13 again differed substantially, while the other two strains clustered together:

So codon analysis also did not reveal any obvious signs of lab origins, but once again confirmed the uniqueness of CoV2 and RaTG13. What does this leave us with? So far, just a number of oddities, which, as scientists like to say, taken together, do not allow us to reject the lab origin hypothesis of CoV2.
The Nature Paper vs. the Lab-Made Hypothesis
But didn’t that Nature article refute the lab-made hypothesis? No, not really. There is no irrefutable evidence against it in the paper, just a loud “we don’t believe so” based on a shaky foundation. Judge for yourself — here are the authors’ key arguments in support of their conclusions:
While the analyses above suggest that SARS-CoV-2 may bind human ACE2 with high affinity, computational analyses predict that the interaction is not ideal and that the RBD sequence is different from those shown in SARS-CoV to be optimal for receptor binding. Thus, the high-affinity binding of the SARS-CoV-2 spike protein to human ACE2 is most likely the result of natural selection on a human or human-like ACE2 that permits another optimal binding solution to arise. This is strong evidence that SARS-CoV-2 is not the product of purposeful manipulation.
In the original paper, the quoted sentences are just below the diagram showing identical RBMs between CoV2 and pangolin-2019. So I am puzzled as to what “computational analysis” has to do with anything. Obviously, the most likely scenario for the lab-made hypothesis is the transfer of RBM from one strain to another — which virologists have done many times before. Therefore, the author’s chain of arguments does not make sense: “computer says binding is not ideal, thus CoV2 must be the result of natural selection. Ergo, this is strong evidence that CoV2 is not lab-made.” Wait, just because CoV2 differs from some “optimal” virus, doesn’t mean it could not have been created in a lab. Not the lab trying to create “optimal” bioweapons, but a lab creating chimeras of naturally found strains, say, in bats and pangolins.
The authors continue to surprise:
Furthermore, if genetic manipulation had been performed, one of the several reverse-genetic systems available for betacoronaviruses would probably have been used. However, the genetic data irrefutably show that SARS-CoV-2 is not derived from any previously used virus backbone.
Again, the same questionable logic dressed in categorical adjectives: “genetic analysis irrefutably proves that CoV2 was not created on the basis of previously known strains!” Well thanks, Captain Obvious. But why couldn’t potential creators of CoV2 make a cDNA backbone from unpublished strains related to or even derived from RaTG13? Then they could easily insert the pangolin RBM into it, as well as add a furin site (or maybe the cDNA backbone already had one). Virologists have been doing things like this for 20 years, and modern genetic engineering tools make such manipulations accessible even to a grad student.
As for the chances of the furin site arising in cell culture, the authors also express strange ideas:
The acquisition of both the polybasic cleavage site and predicted O-linked glycans also argues against culture-based scenarios. New polybasic cleavage sites have been observed only after prolonged passage of low-pathogenicity avian influenza virus in vitro or in vivo. Furthermore, a hypothetical generation of SARS-CoV-2 by cell culture or animal passage would have required prior isolation of a progenitor virus with very high genetic similarity, which has not been described. Subsequent generation of a polybasic cleavage site would have then required repeated passage in cell culture or animals with ACE2 receptors similar to those of humans, but such work has also not previously been described.
First off, the authors themselves cite previous works where the furin site arose in vitro as viruses were cultured in cells. And second, what do they mean, a strain with high genetic similarity has not been described — what about RaTG13? If it had its RBM replaced by one from the pangolin strain, and then the chimeric strain was cultured in vitro, then the furin site could well have arisen in this matter. Additionally, the new strain could thus acquire other mutations that distinguish CoV2 from RaTG13 and pangolin-2019.
But in terms of the potential lab-based origin of the furin site, I am more inclined to hypothesize a specific insertion — as in the Beijing paper from October 2019 with chicken coronavirus. After that, the synthetic strain could have acquired new mutations by subsequent culturing in vitro or in vivo — like the MA15 murine strain in 2007, for example. Or maybe even using the same mouse model with humanized lung tissues and immune system that was created at UNC by Baric’s and other groups in 2018, in which they reported testing several viruses including MERS:
The human innate and adaptive immune system of BLT-L mice
We generated an in vivo model with human lung implants and an autologous human immune system by constructing BLT mice with autologous human lung implants (BLT-L humanized mice).
Finally, even if CoV2 is the product of selection rather than intelligent design, that does not rule out a lab leak either — selection can happen in the lab just as well, both natural and artificial kinds. Different strains can recombine in research animals or in vitro by design or by chance.
On the 4% Genome Difference between RaTG13 and Cov2
Some critics of the lab-made hypothesis claim that the observed ~4% genetic difference between RaTG13 and CoV2 is too high to have possibly occurred in a lab if RaTG13 itself was used as a backbone. Observed mutation rates for RNA viruses vary widely — from 10⁻⁶ to 10⁻⁴ nucleotides per replication in vitro, and in humans CoV2 seems to mutate at a rate of 25 mutations per year. Thus, the logic goes, it would take years, if not decades, for two strains to diverge by 4%. While that is a valid point, there are several issues with that line of reasoning.
First, in vitro mutation speeds (i.e. per unit of time) are much higher, as you can passage cells much more often than infect new animals. As SARS and MERS in vitro experiments showed, significant mutations might be observed after only a few passages. For example, the 2004 paper reported that only after 600 passages there already was a 2.1% difference in the genomic sequences of spike proteins between the original strain and its progeny:

Moreover, in the presence of some antiviral compounds, such as nucleoside analogs (e.g. ribavirin or remdesivir), mutation rates in RNA viruses can increase even further:
We obtained an estimate of the spontaneous mutation rate of ca. 10⁻⁴ substitutions per site or lower, a value within the typically accepted range for RNA viruses. A roughly threefold increase in mutation rate and a significant shift in mutation spectrum were observed in samples from patients undergoing 6 months of interferon plus ribavirin treatment. This result is consistent with the known in vitro mutagenic effect of ribavirin and suggests that the antiviral effect of ribavirin plus interferon treatment is at least partly exerted through lethal mutagenesis.
So if ancestral CoV2 was being lab-tested to assess how its mutagenesis might affect the efficacy of potential vaccines or antiviral drugs, it could have accumulated mutations at a much higher rate.
But possibly, the biggest problem with the 4% difference argument is that it relies on RaTG13 being exactly what WIV says it is. If we are to seriously consider the lab leak hypothesis, we must concede that it does not make sense to blindly trust the data released by the very lab suspected of the leak. If the leak did occur, as is the premise of the lab hypothesis, then the description of what RaTG13 is could be furthering the goal of covering up the leak.
Again, I am not claiming with certainty that is what is happening here. All I am saying is that this is what could have happened, and we need a lot more evidence before we can reach a definitive conclusion. One thing that could help rule out tampering with RaTG13 is having independent labs sequence the 2013 Yunnan samples that She Zhengli extracted RaTG13 from. WIV must still have them if they re-sequenced RaTG13 in 2020.
Shi Zhengli-2020
As I was writing this post, a fresh paper co-authored by Shi Zhengli came out, in which the authors tested a peptide which they have been studying for some time before against CoV2. That peptide was meant to be a pan-coronavirus inhibitor, and its designed mode of action was to block the fusion of a spike protein with a cell membrane. The authors, of course, mention the new furin site of CoV2, and suggest that it may play an important role in the much more efficient penetration of CoV2 into the cell:
In this study, we have shown that SARS-CoV-2 exhibits much higher capacity of membrane fusion than SARS-CoV, suggesting that the fusion machinery of SARS-CoV-2 is an important target for development of coronavirus fusion inhibitors.
…
Generally, β-B coronaviruses lack the S1/S2 furin-recognition site, and their S proteins are uncleaved in the native state. For example, SARS-CoV enters into the cell mainly via the endosomal membrane fusion pathway where its S protein is cleaved by endosomal cathepsin L and activated. Inducing the S1/S2 furin-recognition site could significantly increase the capacity of SARS-CoV S protein to mediate cellular membrane surface infection.
In this context, I wonder whether the authors have previously conducted experiments on how adding a furin site can alter the effectiveness of their peptide (or other drugs or vaccines) against a given coronavirus.
Not to be outdone, Ralph Baric also joined the race to find drugs against CoV2. As I understand, he and co-authors took data on the effectiveness of their nucleoside analogue (β-D-N4-hydroxycytidine, NHC) against SARS-CoV and MERS that they already had, added some in vitro data on CoV2, and sent off the paper to print. Nucleoside analogues (such as the famous remdesivir) are a fundamentally different approach than Shi Zhengli et al. Here, the authors try to prevent viral replication by giving “defective” letters of the genetic alphabet to virus’ copying machine, while Shi Zhengli and coauthors try to prevent the virus from entering the cell altogether. Theoretically, these approaches could be combined.
This is the End, Beautiful Friend
If you made it here by reading rather than scrolling, mad props to you. Hey, even if you scrolled, that’s cool too, and I apologize for the verbosity. I just didn’t anticipate that the rabbit hole would turn out to be a whole underground cave system. I hope that you found this deep dive into the world of virology interesting and enjoyed the exploration of the lab-made CoV2 hypothesis. In my opinion, the data I have presented, taken together, do not allow us to reject this possibility.
Let me be clear: this does NOT prove that CoV2 was synthesized in the laboratory. Yes, as we have seen above, from a technical standpoint, it would not be difficult for a modern virologist to create such a strain. But there is no direct evidence that anyone did this, and strange coincidences cannot pass for circumstantial evidence. On balance, the current chances against this are still higher than for the natural origins of CoV2. Moreover, even if CoV2 was indeed an unfortunate lab leak, the scientists themselves are not to blame, as they were working within the established international laws and guidelines on such research. Now, those who might be trying to cover up that leak, that’s a different story.
The opposite point is worth repeating too: the inverse hypothesis about the exclusively natural origin of the virus does not yet have strong evidence either. Until intermediate ancestors between RaTG13, pangolin-2019 and CoV2 are found, in whom we could trace the mosaic recombination that we observe in CoV2, the question of its origins remains open.
The story begins in April 2012 when six workers in that same Mojiang mine fell ill from a mystery illness while removing bat faeces. Three of the six subsequently died...
We suggest, first, that inside the miners RaTG13 (or a very similar virus) evolved into SARS-CoV-2, an unusually pathogenic coronavirus highly adapted to humans. Second, that the Shi lab used medical samples taken from the miners and sent to them by Kunming University Hospital for their research. It was this human-adapted virus, now known as SARS-CoV-2, that escaped from the WIV in 2019.
We refer to this COVID-19 origin hypothesis as the Mojiang Miners Passage (MMP) hypothesis.
Passaging is a standard virological technique for adapting viruses to new species, tissues, or cell types. It is normally done by deliberately infecting a new host species or a new host cell type with a high dose of virus. This initial viral infection would ordinarily die out because the host’s immune system vanquishes the ill-adapted virus. But, in passaging, before it does die out a sample is extracted and transferred to a new identical tissue, where viral infection restarts. Done iteratively, this technique (called “serial passaging” or just “passaging”) intensively selects for viruses adapted to the new host or cell type (Herfst et al., 2012).
At first glance RaTG13 is unlikely to have evolved into SARS-CoV-2 since RaTG13 is approximately 1,200 nucleotides (3.8%) different from SARS-CoV-2. Although RaTG13 is the most closely related virus to SARS-CoV-2, this sequence difference still represents a considerable gap. In a media statement evolutionary virologist Edward Holmes has suggested this gap represents 20-50 years of evolution and others have suggested similar figures.
We agree that ordinary rates of evolution would not allow RaTG13 to evolve into SARS-CoV-2 but we also believe that conditions inside the lungs of the miners were far from ordinary. Five major factors specific to the hospitalised miners favoured a very high rate of evolution inside them.
i) When viruses infect new species they typically undergo a period of very rapid evolution because the selection pressure on the invading pathogen is high. The phenomenon of rapid evolution in new hosts is well attested among corona- and other viruses (Makino et al., 1986; Baric et al., 1997; Dudas and Rambaut 2016; Forni et al., 2017).
ii) Judging by their clinical symptoms such as the CT scans, all the miner’s infections were primarily of the lungs. This localisation likely occurred initially because the miners were exerting themselves and therefore inhaling the disturbed bat guano deeply. As miners, they may already have had damaged lung tissues (patient 3 had suspected pneumoconiosis) and/or particulate matter was present that irritated the tissues and may have facilitated initial viral entry.
In contrast, standard coronavirus infections are confined to the throat and upper respiratory tract. They do not normally reach the lungs (Perlman and Netland, 2009). Lungs are far larger tissues by weight (kilos vs grammes) than the upper respiratory tract. There was therefore likely a much larger quantity of virus inside the miners than would be the case in an ordinary coronavirus infection.
Comparing a typical coronavirus respiratory tract infection with the extent of infected lungs in the miners from a purely mathematical point of view indicates the potential scale of this quantitative difference. The human aerodigestive tract is approximately 20cm in length and 5cm in circumference, i.e. approximately 100 cm2 in surface area. The surface area of a human lung ranges from 260,000-680,000 cm2(Hasleton, 1972). The amount of potentially infected tissue in an average lung is therefore approximately 4500-fold greater than that available to a normal coronavirus infection. The amount of virus present in the infected miners, sufficient to hospitalise all of them and kill half of them, was thus proportionately very large.
Evolutionary change is in large part a function of the population size. The lungs of the miners, we suggest, supported a very high viral load leading to proportionately rapid viral evolution.
Furthermore, according to the Master’s thesis, the immune systems of the miners were compromised and remained so even for those discharged. This weakness on the part of the miners may also have encouraged evolution of the virus.
iii) The length of infection experienced by the miners (especially patients 2, 3 and 4) far exceeded that of an ordinary coronavirus infection. From first becoming too sick to work in the mine, patient 2 survived 57 days until he died. Patient 3 survived 120 days after stopping work. Patient 4 survived 117 days and then was discharged as cured. Each had been exposed in the mine for 14 days prior to the onset of severe symptoms; thus each presumably had nascent infections for some time before calling in sick (See Table 2 of the thesis).
In contrast, in ordinary coronavirus infections the viral infection is cleared within about ten to fourteen days after being acquired (Tay et al., 2020). Thus, unlike most sufferers from coronavirus infection, the hospitalised miners had very long-term bouts of disease characterised by a continuous high load of virus. In the cases of patients 3 and 4 their illnesses lasted over 4 months.
iv) Coronaviruses are well known to recombine at very high rates: 10% of all progeny in a cell can be recombinants (Makino et al., 1986; Banner and Lai, 1991; Dudas and Rambaut, 2016). In normal virus evolution the mutation rate and the selection pressure are the main foci of attention. But in the case of a coronavirus adapting to a new host where many mutations distributed all over the genome are required to fully adapt to the new host, the recombination rate is likely to be highly influential in determining the overall speed of adaptation by the virus population (Baric et al., 1997).
Inside the miners a large tissue was simultaneously infected by a population of poorly-adapted viruses, with each therefore under pressure to adapt. Even if the starting population of virus lacked any diversity, many individual viruses would have acquired mutations independently but only recombination would have allowed these mutations to unite in the same genome. To recombine, viruses must be present in the same cell. In such a situation the particularities of lung tissues become potentially important because the existence of airways (bronchial tubes, etc.) allows partially-adapted viruses from independent viral populations to travel to distal parts of the lung (or even the other lung) and encounter other such partially-adapted viruses and populations. This movement around the lungs would likely have resulted in what amounted to a passaging effect without the need for a researcher to infect new tissues. Indeed, in the Master’s thesis the observation is several times made that areas of the lungs of a specific patient would appear to heal even while other parts of the lungs would become infected.
v) There were also a number of unusual things about the bat coronaviruses in the mine. They were abnormally abundant but also there were many different kinds, often causing co-infections of the bats (Ge et al., 2016). Viral co-infections are often more infectious or more pathogenic (Latham and Wilson, 2007).
As the WIV researchers remarked about the bats in the mine:“we observed a high rate of co-infection with two coronavirus species and interspecies infection with the same coronavirus species within or across bat families. These phenomena may be owing to the diversity and high density of bat populations in the same cave, facilitating coronavirus intra- and interspecies transmissions, which may result in recombination and acceleration of coronavirus evolution.” (Ge et al., 2016).
The diversity of coronaviruses in the mine suggests that the miners were similarly exposed and that their illness may potentially have begun as co-infections.
Combining these observations, we propose that the miners’ lungs offered an unprecedented opportunity for accelerated evolution of a highly bat-adapted coronavirus into a highly human-adapted coronavirus and that decades of ordinary coronavirus evolution could easily have been condensed into months. However, we acknowledge that these conditions were unique...
We further know that, on June 27th, 2012, the doctors performed an unexplained thymectomy on patient 4. The thymus is an immune organ that can potentially be removed without greatly harming the patient and it could have contained large quantities of virus. Beyond this the Master’s thesis is unfortunately unclear on the specifics of what sampling was done, for what purpose, and where each particular sample went.
Given the interests of the Shi lab in zoonotic origins of human disease, once such a sample was sent to them, it would have been obvious and straightforward for them to investigate how a virus from bats had managed to infect these miners. Any viruses recoverable from the miners would likely have been viewed by them as a unique natural experiment in human passaging offering unprecedented and otherwise-impossible-to-obtain insights into how bat coronaviruses can adapt to humans.
The logical course of such research would be to sequence viral RNA extracted directly from unfrozen tissue or blood samples and/or to generate live infectious clones for which it would be useful (if not imperative) to amplify the virus by placing it in human cell culture. Either technique could have led to accidental infection of a lab researcher.
Our supposition as to why there was a time lag between sample collection (in 2012/2013) and the COVID-19 outbreak is that the researchers were awaiting BSL-4 lab construction and certification, which was underway in 2013 but delayed until 2018.
We propose that, when frozen samples derived from the miners were eventually opened in the Wuhan lab they were already highly adapted to humans to an extent possibly not anticipated by the researchers. One small mistake or mechanical breakdown could have led directly to the first human infection in late 2019.
Thus, one of the miners, most likely patient 3, or patient 4 (whose thymus was removed), was effectively patient zero of the COVID-19 epidemic. In this scenario, COVID-19 is not an engineered virus; but, equally, if it had not been taken to Wuhan and no further molecular research had been performed or planned for it then the virus would have died out from natural causes, rather than escaped to initiate the COVID-19 pandemic.
-- A Proposed Origin for SARS-CoV-2 and the COVID-19 Pandemic, by Jonathan Latham, PhD and Allison Wilson, PhD
In closing, there is no one better to quote on this matter than Ralph Baric himself:
What is the reservoir species of SARS-CoV-2?
They have not identified the actual reservoir species. Reports show that pangolins are potentially the intermediate host, but pangolin viruses are 88–98% identical to SARS-CoV-2. In comparison, civet and racoon dog strains of SARS coronaviruses were 99.8% identical to SARS-CoV from 2003. In other words, we are talking about a handful of mutations between civet strains, racoon dog strains and human strains in 2003. Pangolins [strains of CoV2] have over 3000 nucleotide changes, no way they are the reservoir species. Absolutely no chance.
So there you have it. It remains possible that the mysterious virus host was a lab:

Bad pun? Sorry, last one.
How I Learned to Hate the GOF
I hope this post is not used to prematurely assign blame or propagate one-sided theories. What I do hope it highlights is the scale of dangerous gain-of-function research that has been and is going on in virology. The Covid-19 pandemic really exposed its huge risks in the face of few benefits: GOF research hasn’t protected us from this outbreak, hasn’t provided us with any effective treatments or vaccines in time to save hundreds of thousands of lives lost to CoV2, and if there is even a 0.1% chance GOF research caused the whole thing, that chance is too high.
- admin
- Site Admin
- Posts: 40066
- Joined: Thu Aug 01, 2013 5:21 am
Re: U.S. government gave $3.7 million grant to Wuhan lab at
The Case Is Building That COVID-19 Had a Lab Origin
by Jonathan Latham, PhD and Allison Wilson, PhD
June 2, 2020
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT
If the public has learned a lesson from the COVID-19 pandemic it is that science does not generate certainty. Do homemade face masks work? What is the death rate of COVID-19? How accurate are the tests? How many people have no symptoms? And so on. Practically the lone undisputed assertion made so far is that all the nearest known genetic relatives of its cause, the Sars-CoV-2 virus, are found in horseshoe bats (Zhou et al., 2020). Therefore, the likely viral reservoir was a bat.
However, most of these ancestor-like bat coronaviruses cannot infect humans (Ge et al., 2013). In consequence, from its beginning, a key question hanging over the pandemic has been: How did a bat RNA virus evolve into a human pathogen that is both virulent and deadly?
The answer almost universally seized upon is that there was an intermediate species. Some animal, perhaps a snake, perhaps a palm civet, perhaps a pangolin, served as a temporary host. This bridging animal would probably have had an ACE2 cellular receptor (the molecule which allows cellular entry of the virus) intermediate in protein sequence (or at least structure) between the bat and the human one (Wan et al., 2020).

Shi Zheng-Li releases a bat
In the press and in the scientific literature, scenarios by which this natural zoonotic transfer might have occurred have been endlessly mulled. Most were fuelled by early findings that many of the earliest COVID-19 cases seem to have occurred in and around Wuhan’s Huanan live animal market. [The latest data are that 14 of the 41 earliest cases, including the first, had no connection to the animal market (Huang et al. 2020)].
Since the two previous coronavirus near-pandemics of SARS (2002-3) and MERS (2012) both probably came from bats and both are thought (but not proven) to have transitioned to humans via intermediate animals (civets and dromedaries respectively), a natural zoonotic pathway is a reasonable first assumption (Andersen et al., 2020).
The idea, as it applied to the original (2002) SARS outbreak, is that the original bat virus infected a civet. The virus then evolved briefly in this animal species, but not enough to cause a civet pandemic, and then was picked up by a human before it died out in civets. In this first human (patient zero) the virus survived, perhaps only barely, but was passed on, marking the first case of human to human transmission. As it was successively passed on in its first few human hosts the virus rapidly evolved, adapting to better infect its new hosts. After a few such tentative transmissions the pandemic proper began.
Perhaps this scenario is approximately how the current COVID-19 pandemic began.
But one other troubling possibility must be dispensed with. It follows from the fact that the epicentre city, Wuhan (pop. 11 million), happens to be the global epicentre of bat coronavirus research (e.g. Hu et al., 2017).
Prompted by this proximity, various researchers and news media, prominently the Washington Post, and with much more data Newsweek, have drawn up a prima facie case that a laboratory origin is a strong possibility (Zhan et al., 2020; Piplani et al., 2020). That is, one of the two labs in Wuhan that has worked on coronaviruses accidentally let a natural virus escape; or, the lab was genetically engineering (or otherwise manipulating) a Sars-CoV-2-like virus which then escaped.
Unfortunately, in the US at least, the question of the pandemic’s origin has become a political football; either an opportunity for Sinophobia or a partisan “blame game“.
But the potential of a catastrophic lab release is not a game and systemic problems of competence and opacity are certainly not limited to China (Lipsitch, 2018). The US Department of Homeland Security (DHS) is currently constructing a new and expanded national Bio and Agro-defense facility in Manhattan, Kansas. DHS has estimated that the 50-year risk (defined as having an economic impact of $9-50 billion) of a release from its lab at 70%.
When a National Research Council committee inspected these DHS estimates they concluded “The committee finds that the risks and costs could well be significantly higher than that“.
A subsequent committee report (NAP, 2012) continued:
China, meanwhile, having opened its first in Wuhan in 2018, is planning to roll out a national network of BSL-4 labs (Yuan, 2019). Like many other countries, it is investing significantly in disease surveillance and collection of viruses from wild animal populations and in high-risk recombinant virus research with Potential Pandemic Pathogens (PPPs).
On May 4th, nations and global philanthropies, meeting in Brussels, committed $7.4 billion to future pandemic preparedness. But the question hanging over all such investments is this: the remit of the Wuhan lab at the centre of the accidental release claims is pandemic preparedness. If the COVID-19 pandemic began there then we need to radically rethink current ideas for pandemic preparation globally. Many researchers already believe we should, for the sake of both safety and effectiveness (Lipsitch and Galvani, 2014; Weiss et al., 2015; Lipsitch, 2018). The worst possible outcome would be for those donated billions to accelerate the arrival of the next pandemic.
Historical lab releases, a brief history
An accidental lab release is not merely a theoretical possibility. In 1977 a laboratory in Russia (or possibly China), most likely while developing a flu vaccine, accidentally released the extinct H1N1 influenza virus (Nakajima et al., 1978). H1N1 went on to become a global pandemic virus. A large proportion of the global population became infected. In this case, deaths were few because the population aged over 20 yrs old had historic immunity to the virus. This episode is not widely known because only recently has this conclusion been formally acknowledged in the scientific literature and the virology community has been reluctant to discuss such incidents (Zimmer and Burke, 2009; Wertheim, 2010). Still, laboratory pathogen escapes leading to human and animal deaths (e.g. smallpox in Britain; equine encephalitis in South America) are common enough that they ought to be much better known (summarised in Furmanski, 2014). Only rarely have these broken out into actual pandemics on the scale of H1N1, which, incidentally, broke out again in 2009/2010 as “Swine flu” causing deaths estimated variously at 3,000 to 200,000 on that occasion (Duggal et al., 2016; Simonsen et al. 2013).
Many scientists have warned that experiments with PPPs, like the smallpox and Ebola and influenza viruses, are inherently dangerous and should be subject to strict limits and oversight (Lipsitch and Galvani, 2014; Klotz and Sylvester, 2014). Even in the limited case of SARS-like coronaviruses, since the quelling of the original SARS outbreak in 2003, there have been six documented SARS disease outbreaks originating from research laboratories, including four in China. These outbreaks caused 13 individual infections and one death (Furmanski, 2014). In response to such concerns the US banned certain classes of experiments, called gain of function (GOF) experiments, with PPPs in 2014, but the ban (actually a funding moratorium) was lifted in 2017.
For these reasons, and also to ensure the effectiveness of future pandemic preparedness efforts, it is a matter of vital international importance to establish whether the laboratory escape hypothesis has credible evidence to support it. This must be done regardless of the problem -- in the US -- of toxic partisan politics and nationalism.
The COVID-19 Wuhan lab escape thesis
The essence of the lab escape theory is that Wuhan is the site of the Wuhan Institute of Virology (WIV), China’s first and only Biosafety Level 4 (BSL-4) facility. (BSL-4 is the highest pathogen security level). The WIV, which added a BSL-4 lab only in 2018, has been collecting large numbers of coronaviruses from bat samples ever since the original SARS outbreak of 2002-2003; including collecting more in 2016 (Hu, et al., 2017; Zhou et al., 2018).
Led by researcher Zheng-Li Shi, WIV scientists have also published experiments in which live bat coronaviruses were introduced into human cells (Hu et al., 2017). Moreover, according to an April 14 article in the Washington Post, US Embassy staff visited the WIV in 2018 and “had grave safety concerns” about biosecurity there. The WIV is just eight miles from the Huanan live animal market that was initially thought to be the site of origin of the COVID-19 pandemic.
Wuhan is also home to a lab called the Wuhan Centers for Disease Prevention and Control (WCDPC). It is a BSL-2 lab that is just 250 metres away from the Huanan market. Bat coronaviruses have in the past been kept at the Wuhan WCDPC lab.
Thus the lab escape theory is that researchers from one or both of these labs may have picked up a Sars-CoV-2-like bat coronavirus on one of their many collecting (aka ‘”virus surveillance”) trips. Or, alternatively, a virus they were studying, passaging, engineering, or otherwise manipulating, escaped.
Scientific assessments of the lab escape theory
On April 17 the Australian Science Media Centre asked four Australian virologists: “Did COVID-19 come from a lab in Wuhan?“
Three (Edward Holmes, Nigel McMillan and Hassan Vally) dismissed the lab escape suggestion and Vally simply labeled it, without elaboration, a “conspiracy”.
The fourth virologist interviewed was Nikolai Petrovsky of Flinders University. Petrovsky first addressed the question of whether the natural zoonosis pathway was viable. He told the Media Centre:
That is to say, the idea of an animal intermediate is speculation. Indeed, no credible viral or animal host intermediaries, either in the form of a confirmed animal host or a plausible virus intermediate, has to-date emerged to explain the natural zoonotic transfer of Sars-CoV-2 to humans (e.g. Zhan et al., 2020).
In addition to Petrovsky’s point, there are two further difficulties with the natural zoonotic transfer thesis (apart from the weak epidemiological association between early cases and the Huanan “wet” market).
The first is that researchers from the Wuhan lab travelled to caves in Yunnan (1,500 Km away) to find horseshoe bats containing SARS-like coronaviruses. To-date, the closest living relative of Sars-CoV-2 yet found comes from Yunnan (Ge et al., 2016). Why would an outbreak of a bat virus therefore occur in Wuhan?
Moreover, China has a population of 1.3 billion. If spillover from the wildlife trade was the explanation, then, other things being equal, the probability of a pandemic starting in Wuhan (pop. 11 million) is less than 1%.
Zheng-Li Shi, the head of bat coronavirus research at WIV, told Scientific American as much:
Wuhan, in short, is a rather unlikely epicentre for a natural zoonotic transfer. In contrast, to suspect that Sars-CoV-2 might have come from the WIV is both reasonable and obvious.
Was Sars-CoV-2 created in a lab?
In his statement, Petrovsky goes on to describe the kind of experiment that, in principle, if done in a lab, would obtain the same result as the hypothesised natural zoonotic transfer–rapid adaptation of a bat coronavirus to a human host.
In other words, Petrovsky believes that current experimental methods could have led to an altered virus that escaped.
Passaging, GOF research, and lab escapes
The experiment mentioned by Petrovsky represents a class of experiments called passaging. Passaging is the placing of a live virus into an animal or cell culture to which it is not adapted and then, before the virus dies out, transferring it to another animal or cell of the same type. Passaging is often done iteratively. The theory is that the virus will rapidly evolve (since viruses have high mutation rates) and become adapted to the new animal or cell type. Passaging a virus, by allowing it to become adapted to its new situation, creates a new pathogen.
The most famous such experiment was conducted in the lab of Dutch researcher Ron Fouchier. Fouchier took an avian influenza virus (H5N1) that did not infect ferrets (or other mammals) and serially passaged it in ferrets. The intention of the experiment was specifically to evolve a PPP. After ten passages the researchers found that the virus had indeed evolved, to not only infect ferrets but to transmit to others in neighbouring cages (Herfst et al., 2012). They had created an airborne ferret virus, a Potential Pandemic Pathogen, and a storm in the international scientific community.
The second class of experiments that have frequently been the recipients of criticism are GOF experiments. In GOF research, a novel virus is deliberately created, either by in vitro mutation or by cutting and pasting together two (or more) viruses. The intention of such reconfigurations is to make viruses more infectious by adding new functions such as increased infectivity or pathogenicity. These novel viruses are then experimented on, either in cell cultures or in whole animals. These are the class of experiments banned in the US from 2014 to 2017.
Some researchers have even combined GOF and passaging experiments by using recombinant viruses in passaging experiments (e.g. Sheahan et al., 2008).
Such experiments all require recombinant DNA techniques and animal or cell culture experiments. But the very simplest hypothesis of how Sars-CoV-2 might have been caused by research is simply to suppose that a researcher from the WIV or the WCDCP became infected during a collecting expedition and passed their bat virus on to their colleagues or family. The natural virus then evolved, in these early cases, into Sars-CoV-2. For this reason, even collecting trips have their critics. Epidemiologist Richard Ebright called them “the definition of insanity“. Handling animals and samples exposes collectors to multiple pathogens and returning to their labs then brings those pathogens back to densely crowded locations.
Was the WIV doing experiments that might release PPPs?
Since 2004, shortly after the original SARS outbreak, researchers from the WIV have been collecting bat coronaviruses in an intensive search for SARS-like pathogens (Li et al., 2005). Since the original collecting trip, many more have been conducted (Ge et al., 2013; Ge et al., 2016; Hu et al., 2017; Zhou et al., 2018).
Petrovsky does not mention it but Zheng-Li Shi’s group at the WIV has already performed experiments very similar to those he describes, using those collected viruses. In 2013 the Shi lab reported isolating an infectious clone of a bat coronavirus that they called WIV-1 (Ge et al., 2013). WIV-1 was obtained by introducing a bat coronavirus into monkey cells, passaging it, and then testing its infectivity in human (HeLa) cell lines engineered to express the human ACE2 receptor (Ge et al., 2013).
In 2014, just before the US GOF research ban went into effect, Zheng-Li Shi of WIV co-authored a paper with the lab of Ralph Baric in North Carolina that performed GOF research on bat coronaviruses (Menachery et al., 2015).
In this particular set of experiments the researchers combined “the spike of bat coronavirus SHC014 in a mouse-adapted SARS-CoV backbone” into a single engineered live virus. The spike was supplied by the Shi lab. They put this bat/human/mouse virus into cultured human airway cells and also into live mice. The researchers observed “notable pathogenesis” in the infected mice (Menachery et al. 2015). The mouse-adapted part of this virus comes from a 2007 experiment in which the Baric lab created a virus called rMA15 through passaging (Roberts et al., 2007). This rMA15 was “highly virulent and lethal” to the mice. According to this paper, mice succumbed to “overwhelming viral infection”.
In 2017, again with the intent of identifying bat viruses with ACE2 binding capabilities, the Shi lab at WIV reported successfully infecting human (HeLa) cell lines engineered to express the human ACE2 receptor with four different bat coronaviruses. Two of these were lab-made recombinant (chimaeric) bat viruses. Both the wild and the recombinant viruses were briefly passaged in monkey cells (Hu et al., 2017).
Together, what these papers show is that: 1) The Shi lab collected numerous bat samples with an emphasis on collecting SARS-like coronavirus strains, 2) they cultured live viruses and conducted passaging experiments on them, 3) members of Zheng-Li Shi’s laboratory participated in GOF experiments carried out in North Carolina on bat coronaviruses, 4) the Shi laboratory produced recombinant bat coronaviruses and placed these in human cells and monkey cells. All these experiments were conducted in cells containing human or monkey ACE2 receptors.
The overarching purpose of such work was to see whether an enhanced pathogen could emerge from the wild by creating one in the lab. (For a very informative technical summary of WIV research into bat coronaviruses and that of their collaborators we recommend this post, written by biotech entrepreneur Yuri Deigin).
It also seems that the Shi lab at WIV intended to do more of such research. In 2013 and again in 2017 Zheng-Li Shi (with the assistance of a non-profit called the EcoHealth Alliance) obtained a grant from the US National Institutes of Health (NIH). The most recent such grant proposed that:
It is hard to overemphasize that the central logic of this grant was to test the pandemic potential of SARS-related bat coronaviruses by making ones with pandemic potential, either through genetic engineering or passaging, or both.
Apart from descriptions in their publications we do not yet know exactly which viruses the WIV was experimenting with but it is certainly intriguing that numerous publications since Sars-CoV-2 first appeared have puzzled over the fact that the SARS-CoV-2 spike protein binds with exceptionally high affinity to the human ACE2 receptor “at least ten times more tightly” than the original SARS (Zhou et al., 2020; Wrapp et al., 2020; Wan et al., 2020; Walls et al., 2020; Letko et al., 2020).
This affinity is all the more remarkable because of the relative lack of fit in modelling studies of the SARS-CoV-2 spike to other species, including the postulated intermediates like snakes, civets and pangolins (Piplani et al., 2020). In this preprint these modellers concluded “This indicates that SARS-CoV-2 is a highly adapted human pathogen”.
Given the research and collection history of the Shi lab at WIV it is therefore entirely plausible that a bat SARS-like cornavirus ancestor of Sars-CoV-2 was trained up on the human ACE2 receptor by passaging it in cells expressing that receptor.
[On June 4 an excellent article in the Bulletin of the Atomic Scientists went further. Pointing out what we had overlooked, that the Shi lab also amplified spike proteins of collected coronaviruses, which would make them available for GOF experimentation (Ge et al., 2016).]
How do viruses escape from high security laboratories?
Pathogen lab escapes take various forms. According to the US Government Accountability Office, a US defense Department laboratory once “inadvertently sent live Bacillus anthracis, the bacterium that causes anthrax, to almost 200 laboratories worldwide over the course of 12 years. The laboratory believed that the samples had been inactivated.” In 2007, Britain experienced a foot and mouth disease outbreak. Its’ origin was a malfunctioning waste disposal system of a BSL-4 laboratory leaking into a stream from which neighbouring cows drank. The disposal system had not been properly maintained (Furmanski, 2014). In 2004 an outbreak of SARS originating from the National Institute of Virology (NIV) in Beijing, China, began, again, with the inadequate inactivation of a viral sample that was then distributed to non-secure parts of the building (Weiss et al., 2015).
Writing for the Bulletin of The Atomic Scientists in February 2019, Lynn Klotz concluded that human error was behind most laboratory incidents causing exposures to pathogens in US high security laboratories. While equipment failure was also a factor, of the 749 incidents reported to the US Federal Select Agent Programme between 2009-2015, Klotz concluded that 79% resulted from human error.
But arguably the biggest worry is incidents that go entirely unreported because escape of the pathogen goes undetected. It is truly alarming that a significant number of pathogen escape events were uncovered only because investigators were in the process of examining a completely different incident (Furmanski, 2014). Such discoveries represent strong evidence that pathogen escapes are under-reported and that important lessons still need to be learned (Weiss et al., 2015).
The safety record of the WIV
The final important data point is the biosafety history of the WIV. The WIV was built in 2015 and became a commissioned BSL-4 lab in 2018. According to Josh Rogin of the Washington Post, US embassy officials visited the WIV in 2018. They subsequently warned their superiors in Washington of a “serious shortage of appropriately trained technicians and investigators needed to safely operate this high-containment laboratory”.
And according to VOA News, a year before the outbreak, “a security review conducted by a Chinese national team found the lab did not meet national standards in five categories.”
Credible reports from within China also question lab biosafety and its management. In 2019, Yuan Zhiming, biosecurity specialist at the WIV, cited the “challenges” of biosafety in China. According to Yuan: “several high-level BSLs have insufficient operational funds for routine yet vital processes” and “Currently, most laboratories lack specialized biosafety managers and engineers.” He recommends that “We should promptly revise the existing regulations, guidelines, norms, and standards of biosafety and biosecurity”. Nevertheless, he also notes that China intends to build “5-7” more BSL-4 laboratories (Yuan, 2019).
And in February 2020, Scientific American interviewed Zheng-Li Shi. Accompanying the interview was a photograph of her releasing a captured bat. In the photo she is wearing a casual pink unzipped top layer, thin gloves, and no face mask or other protection. Yet this is the same researcher whose talks give “chilling” warnings about the dire risks of human contact with bats.
All of which tends to confirm the original State Department assessment. As one anonymous “senior administration official” told Rogin:
“The idea that it was just a totally natural occurrence is circumstantial. The evidence it leaked from a lab is circumstantial. Right now, the ledger on the side of it leaking from the lab is packed with bullet points and there’s almost nothing on the other side.”
The leading hypothesis is a lab outbreak
For all these reasons, a lab escape is by far the leading hypothesis to explain the origins of Sars-CoV-2 and the COVID-19 pandemic. The sheer proximity of the WIV and WCDCP labs to the outbreak and the nature of their work represents evidence that can hardly be ignored. The long international history of lab escapes and the biosafety concerns from all directions about the labs in Wuhan greatly strengthen the case. Especially since evidence for the alternative hypothesis, in the form of a link to wild animal exposure or the wildlife trade, remains extremely weak, being based primarily on analogy with SARS one (Bell et al,. 2004; Andersen et al., 2020).
Nevertheless, on April 16th Peter Daszak, who is the President of the EcoHealth Alliance, told Democracy Now! in a lengthy interview that the lab escape thesis was “Pure baloney”. He told listeners:
“There was no viral isolate in the lab. There was no cultured virus that’s anything related to SARS coronavirus 2. So it’s just not possible.”
Daszak made very similar claims on CNN’s Sixty Minutes: “There is zero evidence that this virus came out of a lab in China.” Instead, Daszak encouraged viewers to blame “hunting and eating wildlife”.
Daszak’s certainty is highly problematic on several counts. The closest related known coronaviruses to Sars-CoV-2 are to be found at the WIV so a lot depends on what he means by “related to”. But it is also dishonest in the sense that Daszak must know that culturing in the lab is not the only way that WIV researchers could have caused an outbreak. Third, and this is not Daszak’s fault, the media are asking the right question to the wrong person.
As alluded to above, Daszak is the named principal investigator on multiple US grants that went to the Shi lab at WIV. He is also a co-author on numerous papers with Zheng-Li Shi, including the 2013 Nature paper announcing the isolation of coronavirus WIV-1 through passaging (Ge et al., 2013). One of his co-authorships is on the collecting paper in which his WIV colleagues placed the four fully functional bat coronaviruses into human cells containing the ACE2 receptor (Hu et al. 2017). That is, Daszak and Shi together are collaborators and co-responsible for most of the published high-risk collecting and experimentation at the WIV.
An investigation is needed, but who will do it?
If the Shi lab has anything to hide, it is not only the Chinese Government that will be reluctant to see an impartial investigation proceed. Much of the work was funded by the US taxpayer, channeled there by Peter Daszak and the EcoHealth Alliance. Virtually every credible international organisation that might in principle carry out such an investigation, the WHO, the US CDC, the FAO, the US NIH, including the Gates Foundation, is either an advisor to, or a partner of, the EcoHealth Alliance. If the Sars-CoV-2 outbreak originated from the bat coronavirus work at the WIV then just about every major institution in the global public health community is implicated.
But to solve many of these questions does not necessarily require an expensive investigation. It would probably be enough to inspect the lab notebooks of WIV researchers. All research scientists keep detailed notes, for intellectual property and other reasons, but especially in BSL-4 labs. As Yuan Zhiming told Nature magazine in an article marking the opening of the facility in Wuhan: “We tell them [staff] the most important thing is that they report what they have or haven’t done.”
Meticulous lab records plus staff health records and incident reports of accidents and near-accidents are all essential components (or should be) of BSL work. Their main purpose is to enable the tracking of actual incidents. Much speculation could be ended with the public release of that information. But the WIV has not provided it.
This is puzzling since the Chinese government has a very strong incentive to produce those records. Complete transparency would potentially dispel the gales of blame coming its way; especially on the question of whether Sars-CoV-2 has an engineered or passaged origin. If Zheng-Li Shi and Peter Daszak are correct that nothing similar to Sars-CoV-2 was being studied there, then those notebooks should definitively exonerate the lab from having knowingly made an Actual Pandemic Pathogen.
Given the simplicity and utility of this step this lack of transparency suggests that there is something to hide. If so, it must be important. But then the question is: What?
A thorough investigation of the WIV and its bat coronavirus research is an important first step. But the true questions are not the specific mishaps and dissemblings of Drs Shi or Daszak, nor of the WIV, nor even of the Chinese government.
Rather, the bigger question concerns the current philosophy of pandemic prediction and prevention. Deep enquiries should be made about the overarching wisdom of plucking and counting viruses from the wild and then performing dangerous ‘what if’ recombinant research in high tech but fallible biosafety labs. This is a reductionistic approach, we also note, that has so far failed to predict or protect us from pandemics and may never do so.
END
Footnote:
This article was updated on June 3rd to broaden the estimates of “Swine Flu” deaths, from 3,000 to 3- to 200,000.
Note: On July 15th we published a follow-up to this article: “A Proposed Origin for SARS-CoV-2 and the COVID-19 Pandemic” which carries the analysis above much further and proposes exactly how Sars-CoV-2 might have escaped from the WIV.
References
Andersen, K. G., Rambaut, A., Lipkin, W. I., Holmes, E. C., & Garry, R. F. (2020). The proximal origin of SARS-CoV-2. Nature medicine, 26(4), 450-452.
Bell, D., Roberton, S., & Hunter, P. R. (2004). Animal origins of SARS coronavirus: possible links with the international trade in small carnivores. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 359(1447), 1107-1114.
Duggal, A., Pinto, R., Rubenfeld, G., & Fowler, R. A. (2016). Global variability in reported mortality for critical illness during the 2009-10 influenza A (H1N1) pandemic: a systematic review and meta-regression to guide reporting of outcomes during disease outbreaks. PloS one, 11(5), e0155044.
Furmanski, M. (2014). Laboratory Escapes and “Self-fulfilling prophecy” Epidemics. Report: Center for Arms Control and Nonproliferation. PDF available online.
Ge, X. Y., Li, J. L., Yang, X. L., Chmura, A. A., Zhu, G., Epstein, J. H., … & Zhang, Y. J. (2013). Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature, 503(7477), 535-538.
Ge, X. Y., Wang, N., Zhang, W., Hu, B., Li, B., Zhang, Y. Z., … & Wang, B. (2016). Coexistence of multiple coronaviruses in several bat colonies in an abandoned mineshaft. Virologica Sinica, 31(1), 31-40.
Hu, B., Zeng, L. P., Yang, X. L., Ge, X. Y., Zhang, W., Li, B., … & Luo, D. S. (2017). Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS pathogens, 13(11), e1006698.
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., … & Cheng, Z. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet, 395(10223), 497-506.
Klotz, L. C., & Sylvester, E. J. (2014). The consequences of a lab escape of a potential pandemic pathogen. Frontiers in public health, 2, 116.
Letko, M., Marzi, A., & Munster, V. (2020). Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature microbiology, 5(4), 562- 569.
Li, W., Shi, Z., Yu, M., Ren, W., Smith, C., Epstein, J. H., … & Zhang, J. (2005). Bats are natural reservoirs of SARS-like coronaviruses. Science, 310(5748), 676-679.
Lipsitch, M. (2018). Why Do Exceptionally Dangerous Gain-of-Function Experiments in Influenza?. In Influenza Virus (pp. 589-608). Humana Press, New York, NY.
Lipsitch, M., & Galvani, A. P. (2014). Ethical alternatives to experiments with novel potential pandemic pathogens. PLoS Med, 11(5), e1001646.
Menachery, V. D., Yount, B. L., Debbink, K., Agnihothram, S., Gralinski, L. E., Plante, J. A., … & Randell, S. H. (2015). A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nature medicine, 21(12), 1508-1513.
Nakajima, K., Desselberger, U., & Palese, P. (1978). Recent human influenza A (H1N1) viruses are closely related genetically to strains isolated in 1950. Nature, 274(5669), 334-339.
National Research Council. (2012). Evaluation of the updated site-specific risk assessment for the national bio-and agro-defense facility in Manhattan, Kansas. National Academies Press.
Piplani, S., Singh, P. K., Winkler, D. A., & Petrovsky, N. (2020). In silico comparison of spike protein-ACE2 binding affinities across species; significance for the possible origin of the SARS- CoV-2 virus. arXiv preprint arXiv:2005.06199.
Roberts, A., Deming, D., Paddock, C. D., Cheng, A., Yount, B., Vogel, L., … & Zaki, S. R. (2007). A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog, 3(1), e5.
Sheahan, T., Rockx, B., Donaldson, E., Sims, A., Pickles, R., Corti, D., & Baric, R. (2008). Mechanisms of zoonotic severe acute respiratory syndrome coronavirus host range expansion in human airway epithelium. Journal of virology, 82(5), 2274-2285.
Simonsen, L., Spreeuwenberg, P., Lustig, R., Taylor, R. J., Fleming, D. M., Kroneman, M., … & Paget, W. J. (2013). Global mortality estimates for the 2009 Influenza Pandemic from the GLaMOR project: a modeling study. PLoS Med, 10(11), e1001558.
Walls, A. C., Park, Y. J., Tortorici, M. A., Wall, A., McGuire, A. T., & Veesler, D. (2020). Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell, 180, 281-292.
Wan, Y., Shang, J., Graham, R., Baric, R. S., & Li, F. (2020). Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. Journal of virology, 94(7).
Weiss, S., Yitzhaki, S., & Shapira, S. C. (2015). Lessons to be Learned from Recent Biosafety Incidents in the United States. The Israel Medical Association Journal: IMAJ, 17(5), 269-273.
Wertheim, J. O. (2010). The re-emergence of H1N1 influenza virus in 1977: a cautionary tale for estimating divergence times using biologically unrealistic sampling dates. PloS one, 5(6), e11184.
Wrapp, D., Wang, N., Corbett, K. S., Goldsmith, J. A., Hsieh, C. L., Abiona, O., … & McLellan, J. S. (2020). Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science, 367(6483), 1260-1263.
Zhan, S. H., Deverman, B. E., & Chan, Y. A. (2020). SARS-CoV-2 is well adapted for humans. What does this mean for re-emergence?. bioRxiv. doi: https://doi.org/10.1101/2020.05.01.073262
Zimmer, S. M., & Burke, D. S. (2009). Historical perspective—emergence of influenza A (H1N1) viruses. New England Journal of Medicine, 361(3), 279-285.
Zhou, P., Fan, H., Lan, T., Yang, X. L., Shi, W. F., Zhang, W., … & Zheng, X. S. (2018). Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature, 556(7700), 255-258.
Zhou, P., Yang, X. L., Wang, X. G., Hu, B., Zhang, L., Zhang, W., … & Chen, H. D. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. nature, 579(7798), 270-273.
by Jonathan Latham, PhD and Allison Wilson, PhD
June 2, 2020
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT
YOU ARE REQUIRED TO READ THE COPYRIGHT NOTICE AT THIS LINK BEFORE YOU READ THE FOLLOWING WORK, THAT IS AVAILABLE SOLELY FOR PRIVATE STUDY, SCHOLARSHIP OR RESEARCH PURSUANT TO 17 U.S.C. SECTION 107 AND 108. IN THE EVENT THAT THE LIBRARY DETERMINES THAT UNLAWFUL COPYING OF THIS WORK HAS OCCURRED, THE LIBRARY HAS THE RIGHT TO BLOCK THE I.P. ADDRESS AT WHICH THE UNLAWFUL COPYING APPEARED TO HAVE OCCURRED. THANK YOU FOR RESPECTING THE RIGHTS OF COPYRIGHT OWNERS.
If the public has learned a lesson from the COVID-19 pandemic it is that science does not generate certainty. Do homemade face masks work? What is the death rate of COVID-19? How accurate are the tests? How many people have no symptoms? And so on. Practically the lone undisputed assertion made so far is that all the nearest known genetic relatives of its cause, the Sars-CoV-2 virus, are found in horseshoe bats (Zhou et al., 2020). Therefore, the likely viral reservoir was a bat.
However, most of these ancestor-like bat coronaviruses cannot infect humans (Ge et al., 2013). In consequence, from its beginning, a key question hanging over the pandemic has been: How did a bat RNA virus evolve into a human pathogen that is both virulent and deadly?
The answer almost universally seized upon is that there was an intermediate species. Some animal, perhaps a snake, perhaps a palm civet, perhaps a pangolin, served as a temporary host. This bridging animal would probably have had an ACE2 cellular receptor (the molecule which allows cellular entry of the virus) intermediate in protein sequence (or at least structure) between the bat and the human one (Wan et al., 2020).

Shi Zheng-Li releases a bat
In the press and in the scientific literature, scenarios by which this natural zoonotic transfer might have occurred have been endlessly mulled. Most were fuelled by early findings that many of the earliest COVID-19 cases seem to have occurred in and around Wuhan’s Huanan live animal market. [The latest data are that 14 of the 41 earliest cases, including the first, had no connection to the animal market (Huang et al. 2020)].
Since the two previous coronavirus near-pandemics of SARS (2002-3) and MERS (2012) both probably came from bats and both are thought (but not proven) to have transitioned to humans via intermediate animals (civets and dromedaries respectively), a natural zoonotic pathway is a reasonable first assumption (Andersen et al., 2020).
The idea, as it applied to the original (2002) SARS outbreak, is that the original bat virus infected a civet. The virus then evolved briefly in this animal species, but not enough to cause a civet pandemic, and then was picked up by a human before it died out in civets. In this first human (patient zero) the virus survived, perhaps only barely, but was passed on, marking the first case of human to human transmission. As it was successively passed on in its first few human hosts the virus rapidly evolved, adapting to better infect its new hosts. After a few such tentative transmissions the pandemic proper began.
Perhaps this scenario is approximately how the current COVID-19 pandemic began.
But one other troubling possibility must be dispensed with. It follows from the fact that the epicentre city, Wuhan (pop. 11 million), happens to be the global epicentre of bat coronavirus research (e.g. Hu et al., 2017).
Prompted by this proximity, various researchers and news media, prominently the Washington Post, and with much more data Newsweek, have drawn up a prima facie case that a laboratory origin is a strong possibility (Zhan et al., 2020; Piplani et al., 2020). That is, one of the two labs in Wuhan that has worked on coronaviruses accidentally let a natural virus escape; or, the lab was genetically engineering (or otherwise manipulating) a Sars-CoV-2-like virus which then escaped.
Unfortunately, in the US at least, the question of the pandemic’s origin has become a political football; either an opportunity for Sinophobia or a partisan “blame game“.
But the potential of a catastrophic lab release is not a game and systemic problems of competence and opacity are certainly not limited to China (Lipsitch, 2018). The US Department of Homeland Security (DHS) is currently constructing a new and expanded national Bio and Agro-defense facility in Manhattan, Kansas. DHS has estimated that the 50-year risk (defined as having an economic impact of $9-50 billion) of a release from its lab at 70%.
When a National Research Council committee inspected these DHS estimates they concluded “The committee finds that the risks and costs could well be significantly higher than that“.
A subsequent committee report (NAP, 2012) continued:
“the committee was instructed to judge the adequacy and validity of the uSSRA [updated Site-Specific Risk Assessment]. The committee has identified serious concerns about (1) the misapplication of methods used to assess risk, (2) the failure to make clear whether and how the evidence used to support risk assessment assumptions had been thoroughly reviewed and adequately evaluated, (3) the limited breadth of literature cited and the misinterpretation of some of the significant supporting literature, (4) the failure to explain the criteria used to select assumptions when supporting literature is conflicting, (5) the failure to consider important risk pathways, and (6) the inadequate treatment of uncertainty. Those deficiencies are not equally problematic, but they occur with sufficient frequency to raise doubts about the adequacy and validity of the risk results presented. In most instances (e.g., operational activities at the NBAF), the identified problems lead to an underestimation of risk; in other instances (e.g., catastrophic natural hazards), the risks may be overestimated. As a result, the committee concludes that the uSSRA is technically inadequate in critical respects and is an insufficient basis on which to judge the risks associated with the proposed NBAF in Manhattan, Kansas.”
China, meanwhile, having opened its first in Wuhan in 2018, is planning to roll out a national network of BSL-4 labs (Yuan, 2019). Like many other countries, it is investing significantly in disease surveillance and collection of viruses from wild animal populations and in high-risk recombinant virus research with Potential Pandemic Pathogens (PPPs).
On May 4th, nations and global philanthropies, meeting in Brussels, committed $7.4 billion to future pandemic preparedness. But the question hanging over all such investments is this: the remit of the Wuhan lab at the centre of the accidental release claims is pandemic preparedness. If the COVID-19 pandemic began there then we need to radically rethink current ideas for pandemic preparation globally. Many researchers already believe we should, for the sake of both safety and effectiveness (Lipsitch and Galvani, 2014; Weiss et al., 2015; Lipsitch, 2018). The worst possible outcome would be for those donated billions to accelerate the arrival of the next pandemic.
Historical lab releases, a brief history
An accidental lab release is not merely a theoretical possibility. In 1977 a laboratory in Russia (or possibly China), most likely while developing a flu vaccine, accidentally released the extinct H1N1 influenza virus (Nakajima et al., 1978). H1N1 went on to become a global pandemic virus. A large proportion of the global population became infected. In this case, deaths were few because the population aged over 20 yrs old had historic immunity to the virus. This episode is not widely known because only recently has this conclusion been formally acknowledged in the scientific literature and the virology community has been reluctant to discuss such incidents (Zimmer and Burke, 2009; Wertheim, 2010). Still, laboratory pathogen escapes leading to human and animal deaths (e.g. smallpox in Britain; equine encephalitis in South America) are common enough that they ought to be much better known (summarised in Furmanski, 2014). Only rarely have these broken out into actual pandemics on the scale of H1N1, which, incidentally, broke out again in 2009/2010 as “Swine flu” causing deaths estimated variously at 3,000 to 200,000 on that occasion (Duggal et al., 2016; Simonsen et al. 2013).
Many scientists have warned that experiments with PPPs, like the smallpox and Ebola and influenza viruses, are inherently dangerous and should be subject to strict limits and oversight (Lipsitch and Galvani, 2014; Klotz and Sylvester, 2014). Even in the limited case of SARS-like coronaviruses, since the quelling of the original SARS outbreak in 2003, there have been six documented SARS disease outbreaks originating from research laboratories, including four in China. These outbreaks caused 13 individual infections and one death (Furmanski, 2014). In response to such concerns the US banned certain classes of experiments, called gain of function (GOF) experiments, with PPPs in 2014, but the ban (actually a funding moratorium) was lifted in 2017.
For these reasons, and also to ensure the effectiveness of future pandemic preparedness efforts, it is a matter of vital international importance to establish whether the laboratory escape hypothesis has credible evidence to support it. This must be done regardless of the problem -- in the US -- of toxic partisan politics and nationalism.
The COVID-19 Wuhan lab escape thesis
The essence of the lab escape theory is that Wuhan is the site of the Wuhan Institute of Virology (WIV), China’s first and only Biosafety Level 4 (BSL-4) facility. (BSL-4 is the highest pathogen security level). The WIV, which added a BSL-4 lab only in 2018, has been collecting large numbers of coronaviruses from bat samples ever since the original SARS outbreak of 2002-2003; including collecting more in 2016 (Hu, et al., 2017; Zhou et al., 2018).
Led by researcher Zheng-Li Shi, WIV scientists have also published experiments in which live bat coronaviruses were introduced into human cells (Hu et al., 2017). Moreover, according to an April 14 article in the Washington Post, US Embassy staff visited the WIV in 2018 and “had grave safety concerns” about biosecurity there. The WIV is just eight miles from the Huanan live animal market that was initially thought to be the site of origin of the COVID-19 pandemic.
Wuhan is also home to a lab called the Wuhan Centers for Disease Prevention and Control (WCDPC). It is a BSL-2 lab that is just 250 metres away from the Huanan market. Bat coronaviruses have in the past been kept at the Wuhan WCDPC lab.
Thus the lab escape theory is that researchers from one or both of these labs may have picked up a Sars-CoV-2-like bat coronavirus on one of their many collecting (aka ‘”virus surveillance”) trips. Or, alternatively, a virus they were studying, passaging, engineering, or otherwise manipulating, escaped.
Scientific assessments of the lab escape theory
On April 17 the Australian Science Media Centre asked four Australian virologists: “Did COVID-19 come from a lab in Wuhan?“
Three (Edward Holmes, Nigel McMillan and Hassan Vally) dismissed the lab escape suggestion and Vally simply labeled it, without elaboration, a “conspiracy”.
The fourth virologist interviewed was Nikolai Petrovsky of Flinders University. Petrovsky first addressed the question of whether the natural zoonosis pathway was viable. He told the Media Centre:
“no natural virus matching to COVID-19 has been found in nature despite an intensive search to find its origins.”
That is to say, the idea of an animal intermediate is speculation. Indeed, no credible viral or animal host intermediaries, either in the form of a confirmed animal host or a plausible virus intermediate, has to-date emerged to explain the natural zoonotic transfer of Sars-CoV-2 to humans (e.g. Zhan et al., 2020).
In addition to Petrovsky’s point, there are two further difficulties with the natural zoonotic transfer thesis (apart from the weak epidemiological association between early cases and the Huanan “wet” market).
The first is that researchers from the Wuhan lab travelled to caves in Yunnan (1,500 Km away) to find horseshoe bats containing SARS-like coronaviruses. To-date, the closest living relative of Sars-CoV-2 yet found comes from Yunnan (Ge et al., 2016). Why would an outbreak of a bat virus therefore occur in Wuhan?
Moreover, China has a population of 1.3 billion. If spillover from the wildlife trade was the explanation, then, other things being equal, the probability of a pandemic starting in Wuhan (pop. 11 million) is less than 1%.
Zheng-Li Shi, the head of bat coronavirus research at WIV, told Scientific American as much:
“I had never expected this kind of thing to happen in Wuhan, in central China.” Her studies had shown that the southern, subtropical provinces of Guangdong, Guangxi and Yunnan have the greatest risk of coronaviruses jumping to humans from animals—particularly bats, a known reservoir. If coronaviruses were the culprit, she remembers thinking, “Could they have come from our lab?”
Wuhan, in short, is a rather unlikely epicentre for a natural zoonotic transfer. In contrast, to suspect that Sars-CoV-2 might have come from the WIV is both reasonable and obvious.
Was Sars-CoV-2 created in a lab?
In his statement, Petrovsky goes on to describe the kind of experiment that, in principle, if done in a lab, would obtain the same result as the hypothesised natural zoonotic transfer–rapid adaptation of a bat coronavirus to a human host.
“Take a bat coronavirus that is not infectious to humans, and force its selection by culturing it with cells that express human ACE2 receptor, such cells having been created many years ago to culture SARS coronaviruses and you can force the bat virus to adapt to infect human cells via mutations in its spike protein, which would have the effect of increasing the strength of its binding to human ACE2, and inevitably reducing the strength of its binding to bat ACE2.
Viruses in prolonged culture will also develop other random mutations that do not affect its function. The result of these experiments is a virus that is highly virulent in humans but is sufficiently different that it no longer resembles the original bat virus. Because the mutations are acquired randomly by selection there is no signature of a human gene jockey, but this is clearly a virus still created by human intervention.”
In other words, Petrovsky believes that current experimental methods could have led to an altered virus that escaped.
Passaging, GOF research, and lab escapes
The experiment mentioned by Petrovsky represents a class of experiments called passaging. Passaging is the placing of a live virus into an animal or cell culture to which it is not adapted and then, before the virus dies out, transferring it to another animal or cell of the same type. Passaging is often done iteratively. The theory is that the virus will rapidly evolve (since viruses have high mutation rates) and become adapted to the new animal or cell type. Passaging a virus, by allowing it to become adapted to its new situation, creates a new pathogen.
The most famous such experiment was conducted in the lab of Dutch researcher Ron Fouchier. Fouchier took an avian influenza virus (H5N1) that did not infect ferrets (or other mammals) and serially passaged it in ferrets. The intention of the experiment was specifically to evolve a PPP. After ten passages the researchers found that the virus had indeed evolved, to not only infect ferrets but to transmit to others in neighbouring cages (Herfst et al., 2012). They had created an airborne ferret virus, a Potential Pandemic Pathogen, and a storm in the international scientific community.
The second class of experiments that have frequently been the recipients of criticism are GOF experiments. In GOF research, a novel virus is deliberately created, either by in vitro mutation or by cutting and pasting together two (or more) viruses. The intention of such reconfigurations is to make viruses more infectious by adding new functions such as increased infectivity or pathogenicity. These novel viruses are then experimented on, either in cell cultures or in whole animals. These are the class of experiments banned in the US from 2014 to 2017.
Some researchers have even combined GOF and passaging experiments by using recombinant viruses in passaging experiments (e.g. Sheahan et al., 2008).
Such experiments all require recombinant DNA techniques and animal or cell culture experiments. But the very simplest hypothesis of how Sars-CoV-2 might have been caused by research is simply to suppose that a researcher from the WIV or the WCDCP became infected during a collecting expedition and passed their bat virus on to their colleagues or family. The natural virus then evolved, in these early cases, into Sars-CoV-2. For this reason, even collecting trips have their critics. Epidemiologist Richard Ebright called them “the definition of insanity“. Handling animals and samples exposes collectors to multiple pathogens and returning to their labs then brings those pathogens back to densely crowded locations.
Was the WIV doing experiments that might release PPPs?
Since 2004, shortly after the original SARS outbreak, researchers from the WIV have been collecting bat coronaviruses in an intensive search for SARS-like pathogens (Li et al., 2005). Since the original collecting trip, many more have been conducted (Ge et al., 2013; Ge et al., 2016; Hu et al., 2017; Zhou et al., 2018).
Petrovsky does not mention it but Zheng-Li Shi’s group at the WIV has already performed experiments very similar to those he describes, using those collected viruses. In 2013 the Shi lab reported isolating an infectious clone of a bat coronavirus that they called WIV-1 (Ge et al., 2013). WIV-1 was obtained by introducing a bat coronavirus into monkey cells, passaging it, and then testing its infectivity in human (HeLa) cell lines engineered to express the human ACE2 receptor (Ge et al., 2013).
In 2014, just before the US GOF research ban went into effect, Zheng-Li Shi of WIV co-authored a paper with the lab of Ralph Baric in North Carolina that performed GOF research on bat coronaviruses (Menachery et al., 2015).
In this particular set of experiments the researchers combined “the spike of bat coronavirus SHC014 in a mouse-adapted SARS-CoV backbone” into a single engineered live virus. The spike was supplied by the Shi lab. They put this bat/human/mouse virus into cultured human airway cells and also into live mice. The researchers observed “notable pathogenesis” in the infected mice (Menachery et al. 2015). The mouse-adapted part of this virus comes from a 2007 experiment in which the Baric lab created a virus called rMA15 through passaging (Roberts et al., 2007). This rMA15 was “highly virulent and lethal” to the mice. According to this paper, mice succumbed to “overwhelming viral infection”.
In 2017, again with the intent of identifying bat viruses with ACE2 binding capabilities, the Shi lab at WIV reported successfully infecting human (HeLa) cell lines engineered to express the human ACE2 receptor with four different bat coronaviruses. Two of these were lab-made recombinant (chimaeric) bat viruses. Both the wild and the recombinant viruses were briefly passaged in monkey cells (Hu et al., 2017).
Together, what these papers show is that: 1) The Shi lab collected numerous bat samples with an emphasis on collecting SARS-like coronavirus strains, 2) they cultured live viruses and conducted passaging experiments on them, 3) members of Zheng-Li Shi’s laboratory participated in GOF experiments carried out in North Carolina on bat coronaviruses, 4) the Shi laboratory produced recombinant bat coronaviruses and placed these in human cells and monkey cells. All these experiments were conducted in cells containing human or monkey ACE2 receptors.
The overarching purpose of such work was to see whether an enhanced pathogen could emerge from the wild by creating one in the lab. (For a very informative technical summary of WIV research into bat coronaviruses and that of their collaborators we recommend this post, written by biotech entrepreneur Yuri Deigin).
It also seems that the Shi lab at WIV intended to do more of such research. In 2013 and again in 2017 Zheng-Li Shi (with the assistance of a non-profit called the EcoHealth Alliance) obtained a grant from the US National Institutes of Health (NIH). The most recent such grant proposed that:
“host range (i.e. emergence potential) will be tested experimentally using reverse genetics, pseudovirus and receptor binding assays, and virus infection experiments across a range of cell cultures from different species and humanized mice” (NIH project #5R01Al110964-04).
It is hard to overemphasize that the central logic of this grant was to test the pandemic potential of SARS-related bat coronaviruses by making ones with pandemic potential, either through genetic engineering or passaging, or both.
Apart from descriptions in their publications we do not yet know exactly which viruses the WIV was experimenting with but it is certainly intriguing that numerous publications since Sars-CoV-2 first appeared have puzzled over the fact that the SARS-CoV-2 spike protein binds with exceptionally high affinity to the human ACE2 receptor “at least ten times more tightly” than the original SARS (Zhou et al., 2020; Wrapp et al., 2020; Wan et al., 2020; Walls et al., 2020; Letko et al., 2020).
This affinity is all the more remarkable because of the relative lack of fit in modelling studies of the SARS-CoV-2 spike to other species, including the postulated intermediates like snakes, civets and pangolins (Piplani et al., 2020). In this preprint these modellers concluded “This indicates that SARS-CoV-2 is a highly adapted human pathogen”.
Given the research and collection history of the Shi lab at WIV it is therefore entirely plausible that a bat SARS-like cornavirus ancestor of Sars-CoV-2 was trained up on the human ACE2 receptor by passaging it in cells expressing that receptor.
[On June 4 an excellent article in the Bulletin of the Atomic Scientists went further. Pointing out what we had overlooked, that the Shi lab also amplified spike proteins of collected coronaviruses, which would make them available for GOF experimentation (Ge et al., 2016).]
How do viruses escape from high security laboratories?
Pathogen lab escapes take various forms. According to the US Government Accountability Office, a US defense Department laboratory once “inadvertently sent live Bacillus anthracis, the bacterium that causes anthrax, to almost 200 laboratories worldwide over the course of 12 years. The laboratory believed that the samples had been inactivated.” In 2007, Britain experienced a foot and mouth disease outbreak. Its’ origin was a malfunctioning waste disposal system of a BSL-4 laboratory leaking into a stream from which neighbouring cows drank. The disposal system had not been properly maintained (Furmanski, 2014). In 2004 an outbreak of SARS originating from the National Institute of Virology (NIV) in Beijing, China, began, again, with the inadequate inactivation of a viral sample that was then distributed to non-secure parts of the building (Weiss et al., 2015).
Writing for the Bulletin of The Atomic Scientists in February 2019, Lynn Klotz concluded that human error was behind most laboratory incidents causing exposures to pathogens in US high security laboratories. While equipment failure was also a factor, of the 749 incidents reported to the US Federal Select Agent Programme between 2009-2015, Klotz concluded that 79% resulted from human error.
But arguably the biggest worry is incidents that go entirely unreported because escape of the pathogen goes undetected. It is truly alarming that a significant number of pathogen escape events were uncovered only because investigators were in the process of examining a completely different incident (Furmanski, 2014). Such discoveries represent strong evidence that pathogen escapes are under-reported and that important lessons still need to be learned (Weiss et al., 2015).
The safety record of the WIV
The final important data point is the biosafety history of the WIV. The WIV was built in 2015 and became a commissioned BSL-4 lab in 2018. According to Josh Rogin of the Washington Post, US embassy officials visited the WIV in 2018. They subsequently warned their superiors in Washington of a “serious shortage of appropriately trained technicians and investigators needed to safely operate this high-containment laboratory”.
Two years before the novel coronavirus pandemic upended the world, U.S. Embassy officials visited a Chinese research facility in the city of Wuhan several times and sent two official warnings back to Washington about inadequate safety at the lab, which was conducting risky studies on coronaviruses from bats. The cables have fueled discussions inside the U.S. government about whether this or another Wuhan lab was the source of the virus — even though conclusive proof has yet to emerge.
In January 2018, the U.S. Embassy in Beijing took the unusual step of repeatedly sending U.S. science diplomats to the Wuhan Institute of Virology (WIV), which had in 2015 become China’s first laboratory to achieve the highest level of international bioresearch safety (known as BSL-4). WIV issued a news release in English about the last of these visits, which occurred on March 27, 2018. The U.S. delegation was led by Jamison Fouss, the consul general in Wuhan, and Rick Switzer, the embassy’s counselor of environment, science, technology and health. Last week, WIV erased that statement from its website, though it remains archived on the Internet.
What the U.S. officials learned during their visits concerned them so much that they dispatched two diplomatic cables categorized as Sensitive But Unclassified back to Washington. The cables warned about safety and management weaknesses at the WIV lab and proposed more attention and help. The first cable, which I obtained, also warns that the lab’s work on bat coronaviruses and their potential human transmission represented a risk of a new SARS-like pandemic.
“During interactions with scientists at the WIV laboratory, they noted the new lab has a serious shortage of appropriately trained technicians and investigators needed to safely operate this high-containment laboratory,” states the Jan. 19, 2018, cable, which was drafted by two officials from the embassy’s environment, science and health sections who met with the WIV scientists. (The State Department declined to comment on this and other details of the story.)
The Chinese researchers at WIV were receiving assistance from the Galveston National Laboratory at the University of Texas Medical Branch and other U.S. organizations, but the Chinese requested additional help. The cables argued that the United States should give the Wuhan lab further support, mainly because its research on bat coronaviruses was important but also dangerous.
As the cable noted, the U.S. visitors met with Shi Zhengli, the head of the research project, who had been publishing studies related to bat coronaviruses for many years. In November 2017, just before the U.S. officials’ visit, Shi’s team had published research showing that horseshoe bats they had collected from a cave in Yunnan province were very likely from the same bat population that spawned the SARS coronavirus in 2003.
“Most importantly,” the cable states, “the researchers also showed that various SARS-like coronaviruses can interact with ACE2, the human receptor identified for SARS-coronavirus. This finding strongly suggests that SARS-like coronaviruses from bats can be transmitted to humans to cause SARS-like diseases. From a public health perspective, this makes the continued surveillance of SARS-like coronaviruses in bats and study of the animal-human interface critical to future emerging coronavirus outbreak prediction and prevention.”
The research was designed to prevent the next SARS-like pandemic by anticipating how it might emerge. But even in 2015, other scientists questioned whether Shi’s team was taking unnecessary risks. In October 2014, the U.S. government had imposed a moratorium on funding of any research that makes a virus more deadly or contagious, known as “gain-of-function” experiments.
As many have pointed out, there is no evidence that the virus now plaguing the world was engineered; scientists largely agree it came from animals. But that is not the same as saying it didn’t come from the lab, which spent years testing bat coronaviruses in animals, said Xiao Qiang, a research scientist at the School of Information at the University of California at Berkeley.
“The cable tells us that there have long been concerns about the possibility of the threat to public health that came from this lab’s research, if it was not being adequately conducted and protected,” he said.
There are similar concerns about the nearby Wuhan Center for Disease Control and Prevention lab, which operates at biosecurity level 2, a level significantly less secure than the level-4 standard claimed by the Wuhan Insititute of Virology lab, Xiao said. That’s important because the Chinese government still refuses to answer basic questions about the origin of the novel coronavirus while suppressing any attempts to examine whether either lab was involved.
Sources familiar with the cables said they were meant to sound an alarm about the grave safety concerns at the WIV lab, especially regarding its work with bat coronaviruses. The embassy officials were calling for more U.S. attention to this lab and more support for it, to help it fix its problems.
“The cable was a warning shot,” one U.S. official said. “They were begging people to pay attention to what was going on.”
No extra assistance to the labs was provided by the U.S. government in response to these cables. The cables began to circulate again inside the administration over the past two months as officials debated whether the lab could be the origin of the pandemic and what the implications would be for the U.S. pandemic response and relations with China.
Inside the Trump administration, many national security officials have long suspected either the WIV or the Wuhan Center for Disease Control and Prevention lab was the source of the novel coronavirus outbreak. According to the New York Times, the intelligence community has provided no evidence to confirm this. But one senior administration official told me that the cables provide one more piece of evidence to support the possibility that the pandemic is the result of a lab accident in Wuhan.
“The idea that it was just a totally natural occurrence is circumstantial. The evidence it leaked from the lab is circumstantial. Right now, the ledger on the side of it leaking from the lab is packed with bullet points and there’s almost nothing on the other side,” the official said.
As my colleague David Ignatius noted, the Chinese government’s original story — that the virus emerged from a seafood market in Wuhan — is shaky. Research by Chinese experts published in the Lancet in January showed the first known patient, identified on Dec. 1, had no connection to the market, nor did more than one-third of the cases in the first large cluster. Also, the market didn’t sell bats.
Shi and other WIV researchers have categorically denied this lab was the origin for the novel coronavirus. On Feb. 3, her team was the first to publicly report the virus known as 2019-nCoV was a bat-derived coronavirus.
The Chinese government, meanwhile, has put a total lockdown on information related to the virus origins. Beijing has yet to provide U.S. experts with samples of the novel coronavirus collected from the earliest cases. The Shanghai lab that published the novel coronavirus genome on Jan. 11 was quickly shut down by authorities for “rectification.” Several of the doctors and journalists who reported on the spread early on have disappeared.
On Feb. 14, Chinese President Xi Jinping called for a new biosecurity law to be accelerated. On Wednesday, CNN reported the Chinese government has placed severe restrictions requiring approval before any research institution publishes anything on the origin of the novel coronavirus.
The origin story is not just about blame. It’s crucial to understanding how the novel coronavirus pandemic started because that informs how to prevent the next one. The Chinese government must be transparent and answer the questions about the Wuhan labs because they are vital to our scientific understanding of the virus, said Xiao.
We don’t know whether the novel coronavirus originated in the Wuhan lab, but the cable pointed to the danger there and increases the impetus to find out, he said.
“I don’t think it’s a conspiracy theory. I think it’s a legitimate question that needs to be investigated and answered,” he said. “To understand exactly how this originated is critical knowledge for preventing this from happening in the future.”
-- State Department cables warned of safety issues at Wuhan lab studying bat coronaviruses, by Josh Rogin, The Washington Post, April 14, 2020
And according to VOA News, a year before the outbreak, “a security review conducted by a Chinese national team found the lab did not meet national standards in five categories.”
Credible reports from within China also question lab biosafety and its management. In 2019, Yuan Zhiming, biosecurity specialist at the WIV, cited the “challenges” of biosafety in China. According to Yuan: “several high-level BSLs have insufficient operational funds for routine yet vital processes” and “Currently, most laboratories lack specialized biosafety managers and engineers.” He recommends that “We should promptly revise the existing regulations, guidelines, norms, and standards of biosafety and biosecurity”. Nevertheless, he also notes that China intends to build “5-7” more BSL-4 laboratories (Yuan, 2019).
And in February 2020, Scientific American interviewed Zheng-Li Shi. Accompanying the interview was a photograph of her releasing a captured bat. In the photo she is wearing a casual pink unzipped top layer, thin gloves, and no face mask or other protection. Yet this is the same researcher whose talks give “chilling” warnings about the dire risks of human contact with bats.
All of which tends to confirm the original State Department assessment. As one anonymous “senior administration official” told Rogin:
“The idea that it was just a totally natural occurrence is circumstantial. The evidence it leaked from a lab is circumstantial. Right now, the ledger on the side of it leaking from the lab is packed with bullet points and there’s almost nothing on the other side.”
The leading hypothesis is a lab outbreak
For all these reasons, a lab escape is by far the leading hypothesis to explain the origins of Sars-CoV-2 and the COVID-19 pandemic. The sheer proximity of the WIV and WCDCP labs to the outbreak and the nature of their work represents evidence that can hardly be ignored. The long international history of lab escapes and the biosafety concerns from all directions about the labs in Wuhan greatly strengthen the case. Especially since evidence for the alternative hypothesis, in the form of a link to wild animal exposure or the wildlife trade, remains extremely weak, being based primarily on analogy with SARS one (Bell et al,. 2004; Andersen et al., 2020).
Nevertheless, on April 16th Peter Daszak, who is the President of the EcoHealth Alliance, told Democracy Now! in a lengthy interview that the lab escape thesis was “Pure baloney”. He told listeners:
“There was no viral isolate in the lab. There was no cultured virus that’s anything related to SARS coronavirus 2. So it’s just not possible.”
The Chinese government has proudly stated that the WIV “preserves more than 1,500 strains of virus,” the largest collection in Asia of bat and other coronaviruses. (The government statement probably should have said 1,500 isolates rather than “strains.”) The 2019 interview with Shi in Scientific American reports that the WIV had at least hundreds of individual strains. These numbers have been reported by Chinese government authorities, and they are being taken at face value here.
From 2004 on, the WIV published many dozens of partial or full genome sequences of coronaviruses in their collection. On June 1, Daszak and Shi published partial genetic sequences of 781 Chinese bat coronaviruses, more than one-third of which had never been published previously. There are also multiple published records of animal infection research with bat coronaviruses at the WIV. In order to carry out the research program described above, the WIV laboratory needs to use live viruses, and not just RNA fragments. This contradicts two of the assertions, made by some commentators, that Shi worked only with RNA fragments and that her laboratory did not maintain live viruses. On May 24, 2020, the director of the WIV acknowledged that the laboratory did have “three live strains of bat corona viruses on site,” but implied only three. Knowledgeable virologists assume that the number must be much higher, probably hundreds of live viral isolates.
-- Did the SARS-CoV-2 virus arise from a bat coronavirus research program in a Chinese laboratory? Very possibly., by Milton Leitenberg
Daszak made very similar claims on CNN’s Sixty Minutes: “There is zero evidence that this virus came out of a lab in China.” Instead, Daszak encouraged viewers to blame “hunting and eating wildlife”.
Daszak’s certainty is highly problematic on several counts. The closest related known coronaviruses to Sars-CoV-2 are to be found at the WIV so a lot depends on what he means by “related to”. But it is also dishonest in the sense that Daszak must know that culturing in the lab is not the only way that WIV researchers could have caused an outbreak. Third, and this is not Daszak’s fault, the media are asking the right question to the wrong person.
As alluded to above, Daszak is the named principal investigator on multiple US grants that went to the Shi lab at WIV. He is also a co-author on numerous papers with Zheng-Li Shi, including the 2013 Nature paper announcing the isolation of coronavirus WIV-1 through passaging (Ge et al., 2013). One of his co-authorships is on the collecting paper in which his WIV colleagues placed the four fully functional bat coronaviruses into human cells containing the ACE2 receptor (Hu et al. 2017). That is, Daszak and Shi together are collaborators and co-responsible for most of the published high-risk collecting and experimentation at the WIV.
An investigation is needed, but who will do it?
If the Shi lab has anything to hide, it is not only the Chinese Government that will be reluctant to see an impartial investigation proceed. Much of the work was funded by the US taxpayer, channeled there by Peter Daszak and the EcoHealth Alliance. Virtually every credible international organisation that might in principle carry out such an investigation, the WHO, the US CDC, the FAO, the US NIH, including the Gates Foundation, is either an advisor to, or a partner of, the EcoHealth Alliance. If the Sars-CoV-2 outbreak originated from the bat coronavirus work at the WIV then just about every major institution in the global public health community is implicated.
But to solve many of these questions does not necessarily require an expensive investigation. It would probably be enough to inspect the lab notebooks of WIV researchers. All research scientists keep detailed notes, for intellectual property and other reasons, but especially in BSL-4 labs. As Yuan Zhiming told Nature magazine in an article marking the opening of the facility in Wuhan: “We tell them [staff] the most important thing is that they report what they have or haven’t done.”
Meticulous lab records plus staff health records and incident reports of accidents and near-accidents are all essential components (or should be) of BSL work. Their main purpose is to enable the tracking of actual incidents. Much speculation could be ended with the public release of that information. But the WIV has not provided it.
This is puzzling since the Chinese government has a very strong incentive to produce those records. Complete transparency would potentially dispel the gales of blame coming its way; especially on the question of whether Sars-CoV-2 has an engineered or passaged origin. If Zheng-Li Shi and Peter Daszak are correct that nothing similar to Sars-CoV-2 was being studied there, then those notebooks should definitively exonerate the lab from having knowingly made an Actual Pandemic Pathogen.
Given the simplicity and utility of this step this lack of transparency suggests that there is something to hide. If so, it must be important. But then the question is: What?
A thorough investigation of the WIV and its bat coronavirus research is an important first step. But the true questions are not the specific mishaps and dissemblings of Drs Shi or Daszak, nor of the WIV, nor even of the Chinese government.
Rather, the bigger question concerns the current philosophy of pandemic prediction and prevention. Deep enquiries should be made about the overarching wisdom of plucking and counting viruses from the wild and then performing dangerous ‘what if’ recombinant research in high tech but fallible biosafety labs. This is a reductionistic approach, we also note, that has so far failed to predict or protect us from pandemics and may never do so.
END
Footnote:
This article was updated on June 3rd to broaden the estimates of “Swine Flu” deaths, from 3,000 to 3- to 200,000.
Note: On July 15th we published a follow-up to this article: “A Proposed Origin for SARS-CoV-2 and the COVID-19 Pandemic” which carries the analysis above much further and proposes exactly how Sars-CoV-2 might have escaped from the WIV.
References
Andersen, K. G., Rambaut, A., Lipkin, W. I., Holmes, E. C., & Garry, R. F. (2020). The proximal origin of SARS-CoV-2. Nature medicine, 26(4), 450-452.
Bell, D., Roberton, S., & Hunter, P. R. (2004). Animal origins of SARS coronavirus: possible links with the international trade in small carnivores. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 359(1447), 1107-1114.
Duggal, A., Pinto, R., Rubenfeld, G., & Fowler, R. A. (2016). Global variability in reported mortality for critical illness during the 2009-10 influenza A (H1N1) pandemic: a systematic review and meta-regression to guide reporting of outcomes during disease outbreaks. PloS one, 11(5), e0155044.
Furmanski, M. (2014). Laboratory Escapes and “Self-fulfilling prophecy” Epidemics. Report: Center for Arms Control and Nonproliferation. PDF available online.
Ge, X. Y., Li, J. L., Yang, X. L., Chmura, A. A., Zhu, G., Epstein, J. H., … & Zhang, Y. J. (2013). Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature, 503(7477), 535-538.
Ge, X. Y., Wang, N., Zhang, W., Hu, B., Li, B., Zhang, Y. Z., … & Wang, B. (2016). Coexistence of multiple coronaviruses in several bat colonies in an abandoned mineshaft. Virologica Sinica, 31(1), 31-40.
Hu, B., Zeng, L. P., Yang, X. L., Ge, X. Y., Zhang, W., Li, B., … & Luo, D. S. (2017). Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS pathogens, 13(11), e1006698.
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., … & Cheng, Z. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet, 395(10223), 497-506.
Klotz, L. C., & Sylvester, E. J. (2014). The consequences of a lab escape of a potential pandemic pathogen. Frontiers in public health, 2, 116.
Letko, M., Marzi, A., & Munster, V. (2020). Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature microbiology, 5(4), 562- 569.
Li, W., Shi, Z., Yu, M., Ren, W., Smith, C., Epstein, J. H., … & Zhang, J. (2005). Bats are natural reservoirs of SARS-like coronaviruses. Science, 310(5748), 676-679.
Lipsitch, M. (2018). Why Do Exceptionally Dangerous Gain-of-Function Experiments in Influenza?. In Influenza Virus (pp. 589-608). Humana Press, New York, NY.
Lipsitch, M., & Galvani, A. P. (2014). Ethical alternatives to experiments with novel potential pandemic pathogens. PLoS Med, 11(5), e1001646.
Menachery, V. D., Yount, B. L., Debbink, K., Agnihothram, S., Gralinski, L. E., Plante, J. A., … & Randell, S. H. (2015). A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nature medicine, 21(12), 1508-1513.
Nakajima, K., Desselberger, U., & Palese, P. (1978). Recent human influenza A (H1N1) viruses are closely related genetically to strains isolated in 1950. Nature, 274(5669), 334-339.
National Research Council. (2012). Evaluation of the updated site-specific risk assessment for the national bio-and agro-defense facility in Manhattan, Kansas. National Academies Press.
Piplani, S., Singh, P. K., Winkler, D. A., & Petrovsky, N. (2020). In silico comparison of spike protein-ACE2 binding affinities across species; significance for the possible origin of the SARS- CoV-2 virus. arXiv preprint arXiv:2005.06199.
Roberts, A., Deming, D., Paddock, C. D., Cheng, A., Yount, B., Vogel, L., … & Zaki, S. R. (2007). A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog, 3(1), e5.
Sheahan, T., Rockx, B., Donaldson, E., Sims, A., Pickles, R., Corti, D., & Baric, R. (2008). Mechanisms of zoonotic severe acute respiratory syndrome coronavirus host range expansion in human airway epithelium. Journal of virology, 82(5), 2274-2285.
Simonsen, L., Spreeuwenberg, P., Lustig, R., Taylor, R. J., Fleming, D. M., Kroneman, M., … & Paget, W. J. (2013). Global mortality estimates for the 2009 Influenza Pandemic from the GLaMOR project: a modeling study. PLoS Med, 10(11), e1001558.
Walls, A. C., Park, Y. J., Tortorici, M. A., Wall, A., McGuire, A. T., & Veesler, D. (2020). Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell, 180, 281-292.
Wan, Y., Shang, J., Graham, R., Baric, R. S., & Li, F. (2020). Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. Journal of virology, 94(7).
Weiss, S., Yitzhaki, S., & Shapira, S. C. (2015). Lessons to be Learned from Recent Biosafety Incidents in the United States. The Israel Medical Association Journal: IMAJ, 17(5), 269-273.
Wertheim, J. O. (2010). The re-emergence of H1N1 influenza virus in 1977: a cautionary tale for estimating divergence times using biologically unrealistic sampling dates. PloS one, 5(6), e11184.
Wrapp, D., Wang, N., Corbett, K. S., Goldsmith, J. A., Hsieh, C. L., Abiona, O., … & McLellan, J. S. (2020). Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science, 367(6483), 1260-1263.
Zhan, S. H., Deverman, B. E., & Chan, Y. A. (2020). SARS-CoV-2 is well adapted for humans. What does this mean for re-emergence?. bioRxiv. doi: https://doi.org/10.1101/2020.05.01.073262
Zimmer, S. M., & Burke, D. S. (2009). Historical perspective—emergence of influenza A (H1N1) viruses. New England Journal of Medicine, 361(3), 279-285.
Zhou, P., Fan, H., Lan, T., Yang, X. L., Shi, W. F., Zhang, W., … & Zheng, X. S. (2018). Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature, 556(7700), 255-258.
Zhou, P., Yang, X. L., Wang, X. G., Hu, B., Zhang, L., Zhang, W., … & Chen, H. D. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. nature, 579(7798), 270-273.
- admin
- Site Admin
- Posts: 40066
- Joined: Thu Aug 01, 2013 5:21 am
Re: U.S. government gave $3.7 million grant to Wuhan lab at
260 campers and staff infected with COVID-19 at Georgia YMCA camp
by KHQ.com
August 1, 2020
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT

The YMCA says it regrets opening a camp in Georgia this summer where 260 campers and staff were infected with COVID-19.
That outbreak happened at Camp High Harbor about 100 miles north of Atlanta.
The CDC reports the virus spread quickly among the campers in just a few days.
The YMCA says it tried to follow safety precautions.
When staffers reported to camp in mid-June, they all tested negative within the previous 12 days.
However, on June 24th, a teen staffer felt sick and tested positive and then the camp started sending all the children home.
It is unclear if any campers or staff have required hospitalization.
by KHQ.com
August 1, 2020
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT
YOU ARE REQUIRED TO READ THE COPYRIGHT NOTICE AT THIS LINK BEFORE YOU READ THE FOLLOWING WORK, THAT IS AVAILABLE SOLELY FOR PRIVATE STUDY, SCHOLARSHIP OR RESEARCH PURSUANT TO 17 U.S.C. SECTION 107 AND 108. IN THE EVENT THAT THE LIBRARY DETERMINES THAT UNLAWFUL COPYING OF THIS WORK HAS OCCURRED, THE LIBRARY HAS THE RIGHT TO BLOCK THE I.P. ADDRESS AT WHICH THE UNLAWFUL COPYING APPEARED TO HAVE OCCURRED. THANK YOU FOR RESPECTING THE RIGHTS OF COPYRIGHT OWNERS.

The YMCA says it regrets opening a camp in Georgia this summer where 260 campers and staff were infected with COVID-19.
That outbreak happened at Camp High Harbor about 100 miles north of Atlanta.
The CDC reports the virus spread quickly among the campers in just a few days.
The YMCA says it tried to follow safety precautions.
When staffers reported to camp in mid-June, they all tested negative within the previous 12 days.
However, on June 24th, a teen staffer felt sick and tested positive and then the camp started sending all the children home.
It is unclear if any campers or staff have required hospitalization.
- admin
- Site Admin
- Posts: 40066
- Joined: Thu Aug 01, 2013 5:21 am
Re: U.S. government gave $3.7 million grant to Wuhan lab at
Part 1 of 2
Is there a Role for the States Parties to the BWC in Oversight of Lab-created Potential Pandemic Pathogens?
by Lynn C. Klotz, PhD
Senior Science Fellow
Center for Arms Control and Non-proliferation
Scientists Working Group on Biological and Chemical Security http://armscontrolcenter.org/issue-cent ... l-weapons/
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT
Summary
Research by Ron Fouchier and Yoshihiro Kawaoka marked the beginning of a “Research Enterprise” creating mammalian-airborne-transmissible highly-pathogenic avian-influenza viruses. For the sake of brevity, they will be called matHPAI. At present, likely more than ten laboratories are creating or researching matHPAI live viruses. While most of our concern has focused on matHPAI, the recent de novo creation of horsepox virus, an orthopoxvirus related to smallpox virus, is also of highly worrisome.
Both these viruses are examples of lab-created potential pandemic pathogens (PPPs), which bring up questions reflecting our concerns: Should details of this dual use research be published? Could lab-created PPPs be accidentally released from a laboratory and seed a human pandemic? Could they be employed as biological weapons?
The probability of accidental release into the community from one of the laboratories in the matHPAI Research Enterprise is uncomfortably high. For these and other lab-created PPPs, just one or a few laboratory-infected researchers could seed an outbreak or a pandemic. Concern over a pandemic from a Research Enterprise laboratory release should rival our grave concern over a natural pandemic as the likelihood of both are similar. Furthermore, a laboratory worker with hostile intent could introduce a PPP into the community.
This is not a problem for future consideration, it is upon us now. There is urgent need for international oversight and regulation of this research.
The BWC States Parties may not believe it to be within the BWC mandate to oversee academic research whose goal is public health. However, if the Parties decide this is within its mandate under Article XII of the BWC, it could speed up the enactment of guidelines and regulations. At the very least, the BWC Parties could and should be the catalyst to launch discussions for a different international treaty on oversight and regulation of this dangerous research, perhaps even banning some research. In the meantime, since enacting new treaties is an uncertain and long process, the BWC Parties should work to pass legislation in their own nations.
Background and Commentary
In 2012, Fouchier published1 the creation of mammalian aerosol-transmissible H5N1 avian influenza virus (matH5N1). This virus is responsible for bird flu outbreaks in Asia, and it kills 60% of poultry workers who become infected through close contact with infected poultry.
The Fouchier research along with that of Kawaoka2 marked the beginning of the “Research Enterprise” for creating matPPPs in the laboratory. Subsequently in 2013, letters to the journals Science and Nature, 3,4 ...
twenty-two virologists notified the research community of their interest in creating airborne-transmissible strains of the also deadly H7N9 Asian influenza virus.
A 2015 commentary5 submitted to the U. S. National Science Advisory Board for Biosecurity (NSABB) identified at least 35 publications from laboratories, mostly in Asia, where matHPAI and other influenza viruses were created or researched. Now, there is likely more published research, and many unpublished research projects are likely underway.
(1) Should details of this dual use research be published?
The methods to create these airborne-transmissible viruses are straight-forward and could be reproduced by researchers not highly skilled in molecular virology. Furthermore, skilled molecular virologists could re-create these viruses by directly making the genetic modifications in the laboratory. Re-creating matHPAI and other PPPs brings up the serious biosecurity concern of their use for hostile purposes.
Criteria6, established in 1982, for making decisions about publication of dual use research,...
have been applied recently by Relman7 to lab-created PPPs. The criteria as described by Relman are:
For some matHPAIs, the dual use concern is now moot, as details needed for airborne transmission in mammals have already been published.
The recent publication providing the details of the de novo creation of horsepox virus is of great concern, as the methods could be used to resurrect the smallpox virus. Smallpox ravaged the world until it was eliminated in 1980. As Koblentz has pointed out8: “The synthesis of horsepox virus takes the world one step closer to the reemergence of smallpox as a threat to global health security.” The international community must do whatever is possible to prevent the reemergence of smallpox.
(2) Could a release from the laboratory into the community seed a pandemic?
A calculation9 of the probability of release from a single lab in the Research Enterprise in a single year was found to be 0.20%. For ten labs in the Research Enterprise carrying out research for ten years, the probability of release from one of the labs is about 10 x 10 x 0.20% = 20%, an uncomfortably high number.
Lipsitch10 and Merler11 estimate the probability of a pandemic from a laboratory release ranges from 5% to 50%. Using an intermediate value in that range, 25% or 0.25, the probability of a pandemic in ten years from the Research Enterprise is the probability of release times the probability that a release leads to a pandemic, which is 0.25 x 20% x 0.25 = 5%. The likelihood of a natural pandemic in the next ten years is about 31% 12. Therefore, concern over a pandemic from a Research Enterprise laboratory release should rival our grave concern over a natural pandemic.
Is there a Role for the States Parties to the BWC in Oversight of Lab-created Potential Pandemic Pathogens?
by Lynn C. Klotz, PhD
Senior Science Fellow
Center for Arms Control and Non-proliferation
Scientists Working Group on Biological and Chemical Security http://armscontrolcenter.org/issue-cent ... l-weapons/
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT
YOU ARE REQUIRED TO READ THE COPYRIGHT NOTICE AT THIS LINK BEFORE YOU READ THE FOLLOWING WORK, THAT IS AVAILABLE SOLELY FOR PRIVATE STUDY, SCHOLARSHIP OR RESEARCH PURSUANT TO 17 U.S.C. SECTION 107 AND 108. IN THE EVENT THAT THE LIBRARY DETERMINES THAT UNLAWFUL COPYING OF THIS WORK HAS OCCURRED, THE LIBRARY HAS THE RIGHT TO BLOCK THE I.P. ADDRESS AT WHICH THE UNLAWFUL COPYING APPEARED TO HAVE OCCURRED. THANK YOU FOR RESPECTING THE RIGHTS OF COPYRIGHT OWNERS.
Summary
Research by Ron Fouchier and Yoshihiro Kawaoka marked the beginning of a “Research Enterprise” creating mammalian-airborne-transmissible highly-pathogenic avian-influenza viruses. For the sake of brevity, they will be called matHPAI. At present, likely more than ten laboratories are creating or researching matHPAI live viruses. While most of our concern has focused on matHPAI, the recent de novo creation of horsepox virus, an orthopoxvirus related to smallpox virus, is also of highly worrisome.
Both these viruses are examples of lab-created potential pandemic pathogens (PPPs), which bring up questions reflecting our concerns: Should details of this dual use research be published? Could lab-created PPPs be accidentally released from a laboratory and seed a human pandemic? Could they be employed as biological weapons?
The probability of accidental release into the community from one of the laboratories in the matHPAI Research Enterprise is uncomfortably high. For these and other lab-created PPPs, just one or a few laboratory-infected researchers could seed an outbreak or a pandemic. Concern over a pandemic from a Research Enterprise laboratory release should rival our grave concern over a natural pandemic as the likelihood of both are similar. Furthermore, a laboratory worker with hostile intent could introduce a PPP into the community.
This is not a problem for future consideration, it is upon us now. There is urgent need for international oversight and regulation of this research.
The BWC States Parties may not believe it to be within the BWC mandate to oversee academic research whose goal is public health. However, if the Parties decide this is within its mandate under Article XII of the BWC, it could speed up the enactment of guidelines and regulations. At the very least, the BWC Parties could and should be the catalyst to launch discussions for a different international treaty on oversight and regulation of this dangerous research, perhaps even banning some research. In the meantime, since enacting new treaties is an uncertain and long process, the BWC Parties should work to pass legislation in their own nations.
Background and Commentary
In 2012, Fouchier published1 the creation of mammalian aerosol-transmissible H5N1 avian influenza virus (matH5N1). This virus is responsible for bird flu outbreaks in Asia, and it kills 60% of poultry workers who become infected through close contact with infected poultry.
The Fouchier research along with that of Kawaoka2 marked the beginning of the “Research Enterprise” for creating matPPPs in the laboratory. Subsequently in 2013, letters to the journals Science and Nature, 3,4 ...
Gain-of-function experiments on H7N9
by Ron A. M. Fouchier, Yoshihiro Kawaoka & 20 co-authors
Nature volume 500, pages 150–151(2013)
August 7, 2013
Since the end of March 2013, avian influenza A viruses of the H7N9 subtype have caused more than 130 human cases of infection in China, many of which were severe, resulting in 43 fatalities. Although this A(H7N9) outbreak is now under control, the virus (or one with similar properties) could re-emerge as winter approaches.
To better assess the pandemic threat posed by A(H7N9) viruses, investigators from the NIAID Centers of Excellence in Influenza Research and Surveillance and other expert laboratories in China and elsewhere have characterized the wild-type avian A(H7N9) viruses in terms of host range, virulence and transmission, and are evaluating the effectiveness of antiviral drugs and vaccine candidates. However, to fully assess the potential risk associated with these novel viruses, there is a need for further research, including experiments that may be classified as 'gain of function' (GOF).
Here we outline the aspects of the current situation that most urgently require additional research, our proposed studies, and risk-mitigation strategies.
The A(H7N9) virus haemagglutinin protein has several motifs that are characteristic of mammalian-adapted and human influenza viruses, including mutations that confer human-type receptor binding and enhanced virus replication in mammals. The pandemic risk rises exponentially should these viruses acquire the ability to transmit readily among humans.
Reports indicate that several A(H7N9) viruses from patients who were undergoing antiviral treatment acquired resistance to the primary medical countermeasure — neuraminidase inhibitors (such as oseltamivir, peramivir and zanamivir). Acquisition of resistance to these inhibitors by A(H7N9) viruses could increase the risk of serious outcomes of A(H7N9) virus infections.
The haemagglutinin proteins of A(H7N9) viruses have a cleavage site that is consistent with a low-pathogenic phenotype in birds. In the past, highly pathogenic H7 variants (with basic amino-acid insertions at the cleavage site that enable the spread of the virus to internal organs) have emerged from populations of low-pathogenic strains circulating in domestic gallinaceous poultry.
Normally, epidemiological studies and characterization of viruses from field isolates are used to inform policy decisions regarding public-health responses to a potential pandemic. However, classical epidemiological tracking does not give public-health authorities the time they need to mount an effective response to mitigate the effects of a pandemic virus. To provide information that can assist surveillance activities — thus enabling appropriate public-health preparations to be initiated before a pandemic — experiments that may result in GOF are critical.
Therefore, after review and approval, we propose to perform experiments that may result in GOF (see 'Proposed gain-of-function experiments').
All experiments proposed by influenza investigators are subject to review by institutional biosafety committees. The committees include experts in the fields of infectious disease, immunology, biosafety, molecular biology and public health; also, members of the public represent views from outside the research community. Risk-mitigation plans for working with potentially dangerous influenza viruses, including the 1918 virus and highly pathogenic avian H5N1 viruses, will be applied to conduct GOF experiments with A(H7N9) viruses (see Supplementary Information). Additional reviews may be required by the funding agencies for proposed studies of A(H7N9) viruses.
The recent H5N1 virus-transmission controversy focused on the balance of risks and benefits of conducting research that proved the ability of the H5N1 virus to become transmissible in mammals (see http://www.nature.com/mutantflu). These findings demonstrated the pandemic potential of H5N1 viruses and reinforced the need for continued optimization of pandemic-preparedness measures. Key mutations associated with adaptation to mammals, included in an annotated inventory for mutations in H5N1 viruses developed by the US Centers for Disease Control and Prevention, were identified in human isolates of A(H7N9) viruses. Scientific evidence of the pandemic threat posed by A(H7N9) viruses, based on H5N1 GOF studies, factored in risk assessments by public-health officials in China, the United States and other countries.
Since the H5 transmission papers were published, follow-up scientific studies have contributed to our understanding of host adaptation by influenza viruses, the development of vaccines and therapeutics, and improved surveillance.
Finally, a benefit of the H5N1 controversy has been the increased dialogue regarding laboratory biosafety and dual-use research. The World Health Organization issued laboratory biosafety guidelines for conducting research on H5N1 transmission and, in the United States, additional oversight policies and risk-mitigation practices have been put in place or proposed. Some journals now encourage authors to include biosafety and biosecurity descriptions in their papers, thereby raising the awareness of researchers intending to replicate experiments.
The risk of a pandemic caused by an avian influenza virus exists in nature. As members of the influenza research community, we believe that the avian A(H7N9) virus outbreak requires focused fundamental and applied research conducted by responsible investigators with appropriate facilities and risk-mitigation plans in place. To answer key questions important to public health, research that may result in GOF is necessary and should be done.Box 1: Proposed gain-of-function experiments
• Immunogenicity. To develop more effective vaccines and determine whether genetic changes that confer altered virulence, host range or transmissibility also change antigenicity.
• Adaptation. To assist with risk assessment of the pandemic potential of field strains and evaluate the potential of A(H7N9) viruses to become better adapted to mammals, including determining the ability of these viruses to reassort with other circulating influenza strains.
• Drug resistance. To assess the potential for drug resistance to emerge in circulating viruses, evaluate the genetic stability of mutations conferring drug resistance, and evaluate the efficacy of combination therapy with antiviral therapeutics. Also, to determine whether A(H7N9) viruses could become resistant to available antiviral drugs, and to identify potential resistance mutations that should be monitored during antiviral treatment.
• Transmission. To assess the pandemic potential of circulating strains and perform transmission studies to identify mutations and gene combinations that confer enhanced transmissibility in mammalian models (such as ferrets and guinea pigs).
• Pathogenicity. To aid risk assessment and identify mechanisms, including reassortment and changes to the haemagglutinin cleavage site, that would enable circulating A(H7N9) viruses to become more pathogenic.
Author information
Affiliations
Erasmus Medical Center, Rotterdam, the Netherlands
Ron A. M. Fouchier
University of Wisconsin-Madison, Wisconsin, USA
Yoshihiro Kawaoka
Consortia
20 co-authors
Corresponding authors
Correspondence to Ron A. M. Fouchier or Yoshihiro Kawaoka.
Supplementary Information
Including a full list of co-authors (PDF 820 kb)
Related links
Related links in Nature Research
Limited airborne transmission of H7N9 influenza A virus between ferrets
Avian flu: Extra oversight for H7N9 experiments
H5N1 virus: Transmission studies resume for avian flu
Pause on avian flu transmission studies
Rights and permissions
Reprints and Permissions
About this article
Cite this article
Fouchier, R., Kawaoka, Y. Gain-of-function experiments on H7N9. Nature 500, 150–151 (2013). https://doi.org/10.1038/500150a
Published: 07 August 2013
Issue Date: 08 August 2013
DOI: https://doi.org/10.1038/500150a
twenty-two virologists notified the research community of their interest in creating airborne-transmissible strains of the also deadly H7N9 Asian influenza virus.
A 2015 commentary5 submitted to the U. S. National Science Advisory Board for Biosecurity (NSABB) identified at least 35 publications from laboratories, mostly in Asia, where matHPAI and other influenza viruses were created or researched. Now, there is likely more published research, and many unpublished research projects are likely underway.
(1) Should details of this dual use research be published?
The methods to create these airborne-transmissible viruses are straight-forward and could be reproduced by researchers not highly skilled in molecular virology. Furthermore, skilled molecular virologists could re-create these viruses by directly making the genetic modifications in the laboratory. Re-creating matHPAI and other PPPs brings up the serious biosecurity concern of their use for hostile purposes.
Criteria6, established in 1982, for making decisions about publication of dual use research,...
Scientific Communication and National Security: A report prepared by the Panel on Scientific Communication and National Security Committee on Science, Engineering, and Public Policy
National Academy of Sciences
National Academy of Engineering
Institute of Medicine
NATIONAL ACADEMY PRESS
Washington, D.C. 1982
PREFACE
The use of American science and technology in the rapid increase in Soviet military strength over the past decade has aroused substantial concern in the current administration. This concern has been expressed frequently in recent months by high-ranking officials, who have called for tighter controls on all forms of technology transfer, including communication among scientists by such means as the publication of papers in scientific journals and by face-to-face meetings. In addition, federal agencies have already taken steps to control the flow of data and information from scientific research. These statements and actions have led to rising concern in the U.S. scientific community that such controls might impede scientific progress and its contribution to the national welfare.
In March 1982, discussions among officials of the Academy complex and the Department of Defense led to the creation of the Panel on Scientific Communication and National Security under the aegis of the Committee on Science, Engineering, and Public Policy, a standing committee, to study the question. The charge to the Panel was, generally, to examine the relation between scientific communication1 and national security in light of the growing concern that foreign nations2 are gaining military advantage from such research. It states four major elements, as follows:
• An examination of the national security interests and the interests in free communication in two or three specific fields of science and technology (e.g., cryptology, very high speed integrated circuits, artificial intelligence) to be selected by the study panel in consultation with the Department of Defense. This analysis will include an examination of the extent to which American research has been used in Soviet military programs and, if possible, a consideration of how such information was transferred. In addition, the Panel will assess and compare the contribution to Soviet military strength from the transfer of research information with that arising from other means of technology transfer, such as the Soviet acquisition of American hardware.
• A review—with an emphasis on the International Traffic in Arms Regulations (ITAR) and the Export Administration Regulations (EAR), and a proposed executive order on the classification system—of the principal policy and operational concerns of the respective government agencies, universities, scientific societies, and researchers. (The proprietary concerns of industry will not be considered.) The goal is to identify issues where common agreement exists, to expose those where apparent disagreements are based on misperceptions and misunderstandings, and, perhaps, to narrow and sharpen the issues on which genuine differences exist.
• A rigorous evaluation of critical issues concerning the application of controls on the flow of research information.
• The development of recommendations and conclusions concerning: (i) the intended and proper reach of controls vis-à-vis various categories of science and technology; (ii) areas of science and technology that are or should be outside the operation of controls; (iii) approaches that might provide more certainty and predictability to the regulatory system; and (iv) alternative procedures that might prove acceptable to all of the concerned sectors.
This study has been sponsored by the Department of Defense, the National Science Foundation, the American Association for the Advancement of Science, the American Chemical Society, the American Geophysical Union, and the National Academy of Sciences.3 The Panel, composed of 19 members, includes senior members of university faculties and administrations, former federal agency officials, and leaders in high-technology industrial firms.
At the time the Panel was created, conversations among the Panel chairman, the President of the National Academy of Sciences, and the Under Secretary of Defense for Research and Engineering led to a decision that Panel members would be given security clearance (if they did not already possess it) so that it would be possible for them to receive classified information about technology transfers to other countries. The Panel was subsequently given three secret-level briefings by members of the intelligence community. In addition, a subpanel, comprising six members of the Panel who hold clearance at the highest level, was briefed at two additional meetings.
The Panel has examined the evidence provided at the intelligence briefings and has sought to deal with this information in a way that would eliminate the need to classify this report. The main thrust of the Panel’s findings is completely reflected in this document. However, the Panel has also produced a classified version of the subpanel report based on the secret intelligence information it was given; this statement is available at the Academy to those with the appropriate security clearance.
The Panel invited as participants in its sessions liaison representatives from all the study’s sponsors as well as from the departments of State and Commerce, the Office of Science and Technology Policy, the intelligence community, the Association of American Universities, the Institute of Electrical and Electronics Engineers, and the American Physical Society. Liaison members participated in the Panel’s open sessions and those with the appropriate security clearance attended the Panel’s classified briefings. A list of all those who participated in the Panel’s deliberations is included (see pages 72–76).LIST OF BRIEFERS, CONTRIBUTORS, AND LIAISON REPRESENTATIVES
Briefers
ARTHUR J.ALEXANDER, Associate Head, Economics Department, The Rand Corporation
BETSY ANDERSON, Consular Officer, Bureau of Consular Affairs, Department of State
LEWIS M.BRANSCOMB, Chief Scientist, IBM Corporation
STEPHEN D.BRYEN, Deputy Assistant Secretary, International Economic, Trade and Security Policy, Department of Defense
WILLIAM D.CAREY, Executive Officer, American Association for the Advancement of Science
MICHAEL CIFRINO, Attorney Advisor, Office of the Assistant General Counsel, Department of Defense
W.DONALD COOKE, Vice President for Research, Cornell University
JOHN C.CROWLEY, Director, Federal Relations for Science and Research, Association of American Universities
JAMES DEARLOVE, Chairman, Committee on Exchanges, Technology Transfer Branch; Defense Intelligence Agency, Department of Defense
BOHDAN DENYSYK, Deputy Assistant Secretary, Export Administration, Department of Commerce
ERWIN FRIEDLANDER, Staff Physicist, Lawrence Berkeley Laboratory
ALBERT GORE, JR., Chairman, Investigations and Oversight Subcommittee, Committee on Science and Technology, House of Representatives
WALTER GRANT, Chief, Technology Transfer Branch of the Nuclear Energy and Applied Science Division, Defense Intelligence Agency, Department of Defense
C.DAVID HARTMANN, Executive Secretary, Technology Transfer Intelligence Committee
MARTIN HELLMAN, Professor, Department of Electrical Engineering, Stanford University
CHARLES HORNER, Deputy Assistant Secretary for Science and Technology, Bureau of Oceans and International Environmental and Scientific Affairs, Department of State
BOBBY RAY INMAN, Deputy Director, Central Intelligence Agency
ERNEST B.JOHNSTON, Senior Deputy Assistant Secretary, Bureau of Economic and Business Affairs, Department of State
FRANCIS B.KAPPER, Director, Military Technology Sharing, International Programs and Technology, Office of the Under Secretary of Defense for Research and Engineering, Department of Defense
MICHAEL LORENZO, Deputy Under Secretary of Defense for Research and Engineering (International Programs and Technology), Department of Defense
MICHAEL B.MARKS, Special Assistant to the Under Secretary, Office of the Under Secretary for Security Assistance, Science and Technology, Department of State
RICHARD F.POST, Deputy Associate Director for Physics, Magnetic Fusion Division, Lawrence Livermore National Laboratory
FRANK H.T.RHODES, President, Cornell University
RONALD RIVEST, Professor, Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology
HOWARD E.ROSENBLUM, Deputy Director for Communications Security, National Security Agency
JOSEPH P.SMALDONE, Chief, Arms Licensing Division, Office of Munitions Contol, Department of State
RICHARD SPICER, Intelligence Analyst, Soviet Section, Intelligence Division, Federal Bureau of Investigation
STEPHEN UNGER, Professor, Department of Computer Science, Columbia University
JACK VORONA, Assistant Vice Director for Scientific and Technical Intelligence (International), Defense Intelligence Agency, Department of Defense
DAVID A.WILSON, President’s Executive Assistant, University of California
LEO YOUNG, Director for Research and Technical Information, Office of the Under Secretary of Defense for Research and Engineering, Department of Defense
Contributors
LAURENCE J.ADAMS, Senior Vice President, Martin Marietta Corporation
WAYNE BERT, Munitions Policy Analyst, International Economic, Trade and Security Policy, Department of Defense
JENNIFER SUE BOND, Program Analyst, National Science Foundation
J.FRED BUCY, President, Texas Instruments, Inc.
ALAN M.CAMPBELL, Executive Secretary, U.S.-U.S.S.R. Committee on Cooperation in Physics, Office of International Affairs, National Academy of Sciences
ROSEMARY CHALK, Program Head for Scientific Freedom and Responsibility, American Association for the Advancement of Science
JOHN C.CROWLEY, Director, Federal Relations for Science and Research, Association of American Universities
EDWARD E.DAVID, JR., President, Exxon Research and Engineering Company
CAROLE A.GANZ, International Science Analyst, National Science Foundation
RICHARD L.GARWIN, IBM Fellow, T.J.Watson Research Center, IBM Corporation
S.E.GOODMAN, Professor, Department of Information Systems and Decision Sciences, University of Arizona
RUTH GREENSTEIN, Associate General Counsel, Policy, National Science Foundation
WILLIAM C.HITTINGER, Executive Vice President, RCA Corporation
JEANNE E.HUDSON, Special Assistant, Office of the Director, National Science Foundation
JOHN W.KISER III, Kiser Research, Inc.
RICHARD KRASNOW, Congressional Science Fellow
LAWRENCE C.MITCHELL, Staff Director, Office of International Affairs, National Academy of Sciences
MARTIN E.PACKARD, Assistant to the Board Chairman, Varian Associates
THOMAS O.PAINE, Thomas Paine Associates
HAROLD RELYEA, Analyst, Government Division, Congressional Research Service
LEONARD M.RIESER, Chairperson, Committee on Scientific Freedom and Responsibility, American Association for the Advancement of Science
IAN M.ROSS, President, Bell Laboratories
ROBERT D.SCHMIDT, Vice Chairman of the Board, Control Data Corporation
ROLAND W.SCHMITT, Vice President, Corporate Research and Development, General Electric Company
MICHAEL A.STROSCIO, Special Assistant to the Director of Research and Technical Information, Office of the Under Secretary of Defense for Research and Engineering, Department of Defense
Liaison Representatives
American Academy of Arts and Sciences
HERMAN FESHBACH, Professor, Department of Physics, Massachusetts Institute of Technology
American Association for the Advancement of Science
J.THOMAS RATCHFORD, Associate Executive Officer, American Association for the Advancement of Science
American Chemical Society
RAYMOND P.MARIELLA, Executive Director, American Chemical Society
American Geophysical Union
FRED SPILHAUS, Executive Director, American Geophysical Union
American Physical Society
MELVIN B.GOTTLIEB, Science and Public Policy Fellow, The Brookings Institution
THOMAS A.BARTLETT, President, Association of American Universities
Association of American Universities-Department of Defense Forum
DAVID A.WILSON, President’s Executive Assistant, University of California
Department of Commerce
BOHDAN DENYSYK, Deputy Assistant Secretary, Export Administration, Department of Commerce
Department of Defense
STEPHEN D.BRYEN, Deputy Assistant Secretary, International Economic, Trade and Security Policy, Department of Defense
FRANCIS B.KAPPER, Director, Military Technology Sharing, International Programs and Technology, Office of the Under Secretary of Defense for Research and Engineering, Department of Defense
LEO YOUNG, Director for Research and Technical Information, Office of the Under Secretary of Defense for Research and Engineering, Department of Defense
Department of State
MICHAEL B.MARKS, Special Assistant to the Under Secretary, Office of the Under Secretary for Security Assistance, Science and Technology, Department of State
Intelligence Community
JAN P.HERRING, Chairman, Technology Transfer Intelligence Committee
National Aeronautics and Space Administration
BURTON I.EDELSON, Associate Administrator for Space Science and Applications, NASA
JACK KERREBROCK, Associate Administrator for Office of Aeronautics and Space Technology, NASA
National Science Foundation
DONALD N.LANGENBERG, Deputy Director, National Science Foundation
EDWARD MCGAFFIGAN, Assistant Director for International Affairs, Office of Science and Technology Policy
The Institute of Electrical and Electronics Engineers, Inc.
ROBERT P.BRISKMAN, Assistant Vice President, COMSAT General Corporation
The Panel held three two-day meetings in Washington at which it was briefed by representatives of the departments of Defense, State, and Commerce, and by representatives of the intelligence community, including the Central Intelligence Agency, the Federal Bureau of Investigation, the Defense Intelligence Agency, and the National Security Agency. The Panel also heard presentations by members of the research community and by university representatives. In addition to these briefings, the Rand Corporation prepared an independent analysis of the transfer of sensitive technology from the United States to the Soviet Union.4 To determine the views of scientists and administrators at major research universities, the Panel asked a group of faculty members and administrative officials at Cornell University to prepare a paper incorporating their own views and those of counterparts at other universities (see Working Papers). The Panel also requested and received letters from a group of executives from high-technology industries expressing their views (see Appendix C). The Panel commissioned papers by experts in various aspects of technology transfer and studied the published material on the subject. It examined a few specific scientific areas in some detail.
In order to determine how and where controls might further the national welfare, it is necessary to balance many factors, including the military advantage from controls, their impact on the ability of the research process to serve military, commercial and basic cultural goals, and their effects on the education of students in science and technology. The Panel hopes that this report serves to identify these important issues and to set out recommendations that achieve an appropriate balance.
The Panel is grateful for the assistance provided by the departments of Defense, State, and Commerce, and by the various intelligence agencies. Without their generous help, our task would have been impossible. The liaison representatives of the various departments, agencies, and organizations also contributed to our effort, and we thank them as well. We are also appreciative of the work of the Cornell University committee, which was headed by W.Donald Cooke. We wish to express special thanks to Frank Press, President of the National Academy of Sciences; Courtland Perkins, President of the National Academy of Engineering; and Philip M.Smith, Executive Officer of the National Academy of Sciences for their help and support. I wish to extend my personal thanks to Lawrence McCray, project director, Mitchel Wallerstein, staff consultant, and to Elizabeth Panos, administrative assistant, for their staff support. We are also grateful to Barbara Darr and Allan Hoffman of the COSEPUP staff. Finally, I wish to express my thanks to the individual members of the Panel for their dedicated service in making an early report possible.
Dale R.Corson
Chairman
_______________
Notes:
1. The Panel has concerned itself with scientific communication flowing from a range of research activities embracing basic and applied research and extending over a series of institutions, including universities, industrial laboratories, and government laboratories. A major share of the Panel’s attention has been devoted to university research where no restraints on dissemination of findings—such as restraints to preserve proprietary interests, for example—have existed.
2. The Panel has concentrated its effort primarily on the U.S.-U.S.S.R. relationship, given the level of concern about that problem and the limited time and resources available.
3. The NAS contribution was drawn from funds used for Academy-initiated projects; the funds were provided by the NAS consortium of private foundations. The consortium comprises the Carnegie Corporation of New York, the Charles E.Culpeper Foundation, the William and Flora Hewlett Foundation, the John D. and Catherine T.MacArthur Foundation, the Andrew W.Mellon Foundation, and the Rockefeller Foundation.
4. This paper, among others, is included in the collected working papers used by the Panel. A photocopy is available from the National Academy Press, 2101 Constitution Avenue, N.W., Washington, D.C. 20418.
have been applied recently by Relman7 to lab-created PPPs. The criteria as described by Relman are:
“[Four] criteria to define research for which communication ought to be limited (all of which must be met): (1) research with dual use or military applications, (2) research with a short time to such applications, (3) research when dissemination could give short-term advantage to adversaries, and (4) research when the information was believed not to be already held by adversaries.”
“Inconvenient Truths” in the Pursuit of Scientific Knowledge and Public Health
by David A. Relman
The Journal of Infectious Diseases, Volume 209, Issue 2, 15 January 2014, Pages 170–172, https://doi.org/10.1093/infdis/jit529
Published: 07 October 2013
(See the major article by Barash and Arnon on pages 183–91and Dover et al on pages 192–202,and the editorial commentaries by Popoff on pages 168–9and Hooper and Hirsch on page 167.)
In this issue of The Journal of Infectious Diseases, a group of scientists and physicians from a state public health laboratory present a discovery with important scientific, public health, and security implications, and a difficult dilemma [1, 2]. Their identification of a novel, eighth botulinum neurotoxin (BoNT) from a patient with botulism expands our understanding of Clostridium botulinum and BoNT diversity, C. botulinum evolution, and the pathogenesis of botulism, but it also reveals a significant public health vulnerability. This new toxin, BoNT/H, cannot be neutralized by any of the currently available antibotulinum antisera, which means that we have no effective treatment for this form of botulism. Until anti-BoNT/H antitoxin can be created, shown to be effective, and deployed, both the strain itself and the sequence of this toxin (with which recombinant protein can be easily made) pose serious risks to public health because of the unusually severe, widespread harm that could result from misuse of either [3]. Thus, the dilemma faced by these authors, and by society, revolves around the question, should all of the information from this and similar studies be fully disseminated, motivated by the desire to realize all possible benefits from the discovery, or should dissemination of some or all of the information be restricted, with the goal of diminishing the probability of misuse?
In the early 1980s, Dale Corson, who was president emeritus of Cornell University, led a now-famous study at the US National Academy of Science that culminated in a report entitled Scientific Communication and National Security [4]. The committee explored the growing tension between the principle of openness in science and consequent concerns about national security, which assumed prominence in US public discourse with the development of the atomic bomb and then served as the basis for vigorous debate at the time of their study during the height of the cold war. The report is remembered for having drawn a sharp, “bright” line between scientific information whose communication deserves strict control, that is, national security classification, and information that should be freely disseminated. The committee recommended a long-term national strategy of “security by accomplishment,” to be achieved through a vigorous and open research enterprise. It reminded readers about the “inherent limits on the feasibility and effectiveness of controls,” especially when pursued in the domain of basic scientific research. The persuasive arguments of the Corson committee about a bright line shaped national policy and were cited in President Reagan's National Security Decision Directive 189, which declared in 1985 that “to the maximum extent possible, the products of fundamental research remain unrestricted …[W]here the national security requires control, the mechanism for control of information generated during federally-funded fundamental research … is classification.” [5] This policy has been reaffirmed by subsequent presidential administrations.
What is less well appreciated is that the Corson report also discussed “a small ‘gray area’ of research activities for which limited restrictions short of classification are appropriate” [4]. The Corson committee offered 4 criteria to define research for which communication ought to be limited (all of which must be met): (1) research with dual use or military applications, (2) research with a short time to such applications, (3) research when dissemination could give short-term advantage to adversaries, and (4) research when the information was believed not to be already held by adversaries. They then suggested that classification was not appropriate in all such circumstances, and that there might be other mechanisms of control. As an alternative mechanism, the committee recommended a form of voluntary prepublication control exercised by the investigator. Of interest, given recent political debates, they cited a successful early experiment in voluntary prepublication control for manuscripts dealing with cryptography, involving academia and the National Security Agency.
The Corson “gray area” was largely ignored in subsequent years, in part because there were few concrete and compelling examples of work that might fit in this category and, in part, because the practical aspects of a nonclassification information control mechanism were, and remain, profoundly challenging. Yet, the ongoing revolution in the life sciences now forces us to confront an uncomfortable reality: The same process by which we gain further understanding of biology, invent powerful methods for reengineering genomes and organisms, and derive critical solutions to the problems that ail us and our planet is a path that will predictably generate new and increasingly substantial risks [6]. An important case study in 2012 involved the deliberate engineering of highly pathogenic avian influenza viruses with enhanced properties of transmissibility [7, 8]. The scientific outcome was easily anticipated as it was the stated and intended goal of the investigators, but the risks of the proposed experiments were not widely discussed ahead of time, nor were alternative scientific approaches or risk mitigation strategies. When the National Science Advisory Board for Biosecurity, of which I am a member, initially recommended to the US government, after careful assessment of the risks and benefits, that some of these scientific results not be widely disseminated, the absence of a mechanism, other than classification, for limited distribution of the information confounded policy makers. The need for such a mechanism now deserves renewed serious deliberation and wider discussion, because we are inadequately served by just 2 options, that is, unrestricted dissemination and classification. Some information from life sciences research has an unusually high likelihood of immediately enabling irresponsible or malevolent persons to do grave harm to society, and deserves some control. Yet, classification may not be appropriate, because the burdens of working within the classified environment might hinder needed countermeasure work, or the criteria for national security classification might not be met, such as in the current case where the information is not owned by, produced by or for, or under the control of the US government [9]. A mechanism for short-term, limited distribution is needed while risk mitigation measures are devised (eg, therapies and vaccines), even though the control of distribution will be far from perfect.
The 2 articles in this issue of JID [1, 2] highlight an important alternative mechanism for management of risk in life sciences research, albeit an imperfect one, and take us back to 1982, to Dale Corson and colleagues. The authors of these articles, believing that the sequence information of BoNT/H poses an immediate and unusually serious risk to society, and that the information was unlikely to be already in the hands of those who would seek to do harm, decided to exercise voluntary prepublication control and to withhold this specific information. The more general and less risky aspects of the information were submitted for publication to alert the public to the discovery. This investigator-initiated strategy has merit and deserves careful consideration, but some major caveats should be noted. First, this strategy offers only short-term benefits, as data generated in today's highly interconnected world will inevitably become disseminated. (Corson's “inherent limits on the effectiveness of controls” are even more apparent today.) Given current capabilities in gene synthesis and expression, possession of the sequence is tantamount to possession of the toxin. Therefore, for this strategy to make sense, every effort should be made to exploit this temporary benefit, and to promote immediate, rapid development of an effective countermeasure, that is, anti-BoNT/H antisera. In fact, studies to inform countermeasure development are well under way. Second, this approach poses significant danger to the research enterprise in general if decisions to adopt this approach are made casually, arbitrarily, or frequently. However, I agree with the actions of the authors in this specific case. Although government officials may have participated in a discussion about these papers, to my knowledge relevant stakeholders outside the government were not involved. Going forward, decisions should be based on the best available guidance from experts representing broad, diverse constituencies, including nonscientist representatives of the public, and should be made in a transparent manner.
As more powerful techniques are used to explore the natural world [1, 2] and generate novel biological diversity [7], benefits and risks will both multiply and magnify. And the “gray area” will expand. Voluntary controls may have worked reasonably well for the field of cryptography in the early 1980s because, as the Corson committee remarked, the field was relatively small, its dual use features were obvious, and at the same time, the National Security Agency had high technical competence and an interest in promoting open science. Today's world of the life sciences is much more challenging and consequential.
The life sciences encompass a large number of disciplines and practitioners around the globe, with disparate purposes. Therefore, more expansive, balanced, and dispassionate discussion will be needed, and it must include difficult questions, such as whether there are experiments that should not be undertaken because of disproportionately high risk. In addition, as suggested by Corson et al., we need to make controls more workable, improve the factual basis for decisions on whether and when to exercise such controls, and improve mutual understanding between the government and the scientific community. Finally, for voluntary controls to play a useful role in the management of problematic information in the “gray area,” scientists will first need to recognize their ethical and moral responsibilities to society in the pursuit of knowledge [10]. Scientists have obligations to society that involve more than blind pursuit of information. Like clinicians, scientists have an obligation to do no harm.
Notes
Financial support. This work was supported by the National Institutes of Health (DP1OD000964, R01AI092531, R01GM099534, R01DE023113, U54AI065359), the Doris Duke Charitable Trust, the March of Dimes Foundation, and the Thomas C. and Joan M. Merigan Endowment at Stanford University.
Potential conflicts of interest.
The author is on the board of Seres Health and Novartis Vaccines, and is a consultant for Proctor & Gamble.
The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
1 Barash JR, Arnon SS. A novel strain of Clostridium botulinum that produces type B and type H botulinum toxins, J Infect Dis, 2014, vol. 209 (pg. 183-91)
2 Dover N, Barash JR, Hill KK, Xie G, Arnon SS. Molecular characterization of a novel botulinum neurotoxin type H gene, J Infect Dis, 2014, vol. 209 (pg. 192-202)
3 Arnon SS, Schechter R, Inglesby TV, et al. Botulinum toxin as a biological weapon: medical and public health management, JAMA, 2001, vol. 285 (pg. 1059-70)
4 Panel on Scientific Communication and National SecurityScientific communication and national security., 1982Washington, DCNational Academy Press
5 National Security Decision Directive 189 (NSDD 189)National policy on transfer of scientific, technical and engineering information, 1985Washington, DC:Executive Office of the President of the United States,
6 Relman DA. The biological century: coming to terms with risk in the life sciences, Nat Immunol, 2010, vol. 11 (pg. 275-8)
7 Herfst S, Schrauwen EJ, Linster M, et al. Airborne transmission of influenza A/H5N1 virus between ferrets, Science, 2012, vol. 336 (pg. 1534-41)
8 Imai M, Watanabe T, Hatta M, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets, Nature, 2012, vol. 486 (pg. 420-8)
9 Executive Office of the President of the United StatesClassified national security information, Executive Order 12958., 1995, vol. 60 Washington, DCFederal Register(pg. 19825-43)
Google Scholar
10 Relman DA. The increasingly compelling moral responsibilities of life scientists, The Hastings Cent Rep, 2013, vol. 43 (pg. 34-5)
For some matHPAIs, the dual use concern is now moot, as details needed for airborne transmission in mammals have already been published.
The recent publication providing the details of the de novo creation of horsepox virus is of great concern, as the methods could be used to resurrect the smallpox virus. Smallpox ravaged the world until it was eliminated in 1980. As Koblentz has pointed out8: “The synthesis of horsepox virus takes the world one step closer to the reemergence of smallpox as a threat to global health security.” The international community must do whatever is possible to prevent the reemergence of smallpox.
The De Novo Synthesis of Horsepox Virus: Implications for Biosecurity and Recommendations for Preventing the Reemergence of Smallpox
by Gregory D. Koblentz
Published Online:1 Dec 2017
https://doi.org/10.1089/hs.2017.0061
Abstract
In March 2017, the American biotech company Tonix announced that a Canadian scientist had synthesized horsepox virus as part of a project to develop a safer vaccine against smallpox. The first de novo synthesis of an orthopoxvirus, a closely related group of viruses that includes horsepox and the variola virus that causes smallpox, crosses an important Rubicon in the field of biosecurity. The synthesis of horsepox virus takes the world one step closer to the reemergence of smallpox as a threat to global health security. That threat has been held at bay for the past 40 years by the extreme difficulty of obtaining variola virus and the availability of effective medical countermeasures. The techniques demonstrated by the synthesis of horsepox have the potential to erase both of these barriers. The primary risk posed by this research is that it will open the door to the routine and widespread synthesis of other orthopoxviruses, such as vaccinia, for use in research, public health, and medicine. The normalization and globalization of orthopoxvirus synthesis for these beneficial applications will create a cadre of laboratories and scientists that will also have the capability and expertise to create infectious variola virus from synthetic DNA. Unless the safeguards against the synthesis of variola virus are strengthened, the capability to reintroduce smallpox into the human population will be globally distributed and either loosely or completely unregulated, providing the foundation for a disgruntled or radicalized scientist, sophisticated terrorist group, unscrupulous company, or rogue state to recreate one of humanity's most feared microbial enemies. The reemergence of smallpox—because of a laboratory accident or an intentional release—would be a global health disaster. International organizations, national governments, the DNA synthesis industry, and the synthetic biology community all have a role to play in devising new approaches to preventing the reemergence of smallpox.
(2) Could a release from the laboratory into the community seed a pandemic?
A calculation9 of the probability of release from a single lab in the Research Enterprise in a single year was found to be 0.20%. For ten labs in the Research Enterprise carrying out research for ten years, the probability of release from one of the labs is about 10 x 10 x 0.20% = 20%, an uncomfortably high number.
Lipsitch10 and Merler11 estimate the probability of a pandemic from a laboratory release ranges from 5% to 50%. Using an intermediate value in that range, 25% or 0.25, the probability of a pandemic in ten years from the Research Enterprise is the probability of release times the probability that a release leads to a pandemic, which is 0.25 x 20% x 0.25 = 5%. The likelihood of a natural pandemic in the next ten years is about 31% 12. Therefore, concern over a pandemic from a Research Enterprise laboratory release should rival our grave concern over a natural pandemic.
- admin
- Site Admin
- Posts: 40066
- Joined: Thu Aug 01, 2013 5:21 am
Re: U.S. government gave $3.7 million grant to Wuhan lab at
Part 2 of 2
Are lab-created potential pandemic pathogens biological weapons?
The possibility has been raised that matHPAIs could be used as biological weapons.
For instance, in a 2012 Comment in the science journal Nature13, the NSABB voiced their concern:
Another concerned voice is found in a lead editorial in the journal Science14 by Nobel Laureate Paul Berg:
The phrases “malevolent individuals, organizations or governments,” “intentionally harmful outcomes,” and “sinister motives” describe employment of these lab-created pathogens as biological weapons.
The Biological Weapons Convention15 was written with a focus on military tactical biological weapons, where significant quantities would usually be employed. Article I of the convention speaks to this focus:
For lab-created PPPs, any quantity, however small, could seed an outbreak or pandemic. In this circumstance, development also implies production and stockpiling, since a single vial and one to a few infected individuals is all that is necessary to launch an attack.
From a military tactical point of view, however, lab-created PPPs would not be good biological weapons as they would boomerang back on the attackers, since they are highly transmissible. Nonetheless, a suicidal terrorist group or a desperate State might employ them as a last resort, or threaten to employ them as a means of extortion.
Call for action from the Parties to the BWC
When Fouchier and Kawaoka carried out their research, it was unlikely that biological weapons even crossed their minds. Since that possibility has now been brought up, researchers who are creating PPPs must take into account the biological weapons risk of dual use information and laboratory release of their pathogens. If there is little public-health benefit or little defense rationale for particular research, the Parties to the BWC should question whether it is biological weapons development and act accordingly.
This is a complex issue. The question of what constitutes biological weapons development is complicated. Many biodefense activities of the U.S Department of Homeland Security’s proposed and now abandoned National Biodefense Analysis and Countermeasures Center would be considered biological weapons development. As pointed out in a letter16 in the journal Politics and the Life Sciences: "Taken together, many of the [proposed] activities…— most particularly the ‘‘Store, Stabilize, Package, Disperse’’ sequence and the ‘‘Computational modeling of feasibility, methods, and scale of production’’ item — may constitute development in the guise of threat assessment, and they certainly will be interpreted that way."
Recent articles directed to the Eighth BWC Review Conference (for instance, see here,...
here,...
[url]here[/url], here,...
and here) 17,18,19, 20,21 call for the Parties to intensify their focus on new science and technology that could lead to violations of the BWC. Lab-created PPPs, particularly matHPAI, because they are already present in laboratories around the world, are an urgent focus.
Article XII of the BWC calls for
Hopefully, the States Parties to the BWC will set in motion a process for overseeing relevant new research and technologies. If the Parties decide lab-created PPPs are within its mandate under Article XII of the BWC, it could speed up the enactment of guidelines and regulations. At the very least, the Parties should be the catalyst to launch discussions for a different international treaty on oversight and regulation of creation and research on highly dangerous agents. In the meantime, since enacting new treaties is an uncertain and long process, Parties to the BWC should pass legislation in their own nations.
_______________
Notes:
1 Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012 Jun 22;336(6088):1534-41. doi: 10.1126/science.1213362
2 Masaki Imai, Tokiko Watanabe, Masato Hatta, Subash C. Das, Makoto Ozawa, Kyoko Shinya, Gongxun Zhong, Anthony Hanson, Hiroaki Katsura, Shinji Watanabe, Chengjun Li, Eiryo Kawakami, Shinya Yamada, Maki Kiso, Yasuo Suzuki, Eileen A. Maher, Gabriele Neumann & Yoshihiro Kawaoka. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012 May 2;486(7403):420-8. doi: 10.1038/nature10831
3 Fouchier RA, Kawaoka Y, Cardona C, Compans RW, García-Sastre A, Govorkova EA, et al. Gain of-function experiments on H7N9. Science. 2013 Aug 9;341(6146):612-3. doi: 10.1126/science.341.6146.612
4 Fouchier RA, Kawaoka Y, Cardona C, Compans RW, Fouchier RA, García-Sastre A, et al. Avian flu: gain-of-function experiments on H7N9. Nature. 2013 Aug 8;500(7461):150-1. doi: 10.1038/500150a.
5 Klotz LC. The Potential Pandemic Influenza Research Enterprise. https://armscontrolcenter.org/wpcontent ... ersion.pdf
6 Panel on Scientific Communication and National Security. Committee on Science, Engineering, and Public Policy: National Academy of Sciences, National Academy of Engineering. Institute of Medicine. Scientific Communication and National Security, NATIONAL ACADEMY PRESS Washington, D.C. 1982
7 Relman DA. "Inconvenient truths" in the pursuit of scientific knowledge and public health. J Infect Dis. 2014 Jan 15;209(2):170-2. doi: 10.1093/infdis/jit529. Epub 2013 Oct 7.
8 Koblentz GD. The De Novo Synthesis of Horsepox Virus: Implications for Biosecurity and Recommendati for Preventing the Reemergence of Smallpox. Health Secur. 2017 Aug 24. doi: 10.1089/hs.2017.0061. [Epub ahead of print]
9 Klotz LC1, Sylvester EJ. The consequences of a lab escape of a potential pandemic pathogen. Front Public Health. 2014 Aug 11;2:116. doi: 10.3389/fpubh.2014.00116
10 Lipsitch M1, Cohen T, Cooper B, Robins JM, Ma S, James L, Gopalakrishna G, Chew SK, Tan CC, Samore MH, Fisman D, Murray M. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003 Jun 20;300(5627):1966-70. Epub 2003 May 23. See Figure 4a.
11 Merler S, Ajelli M, Fumanelli L, Vespignani A. Containing the accidental laboratory escape of potential pandemic influenza viruses. BMC Med. 2013 Nov 28;11:252. doi: 10.1186/1741-7015-11-252.
12 The yearly probability of a natural pandemic may be estimated as follows: The number of years that have passed since the beginning of 1920 until September 8, 2017 = 97.77 years. (The proper starting date is 1920, just after the first pandemic, the 1918 pandemic flu. Any other starting date would be arbitrary.) There have been three pandemics since 1920: 1957, 1968 and 2009. Thus, the probability of a pandemic in any one year is (3 pandemics) / (97.7 years) = 0.031 per year. In ten years, the probability of a natural pandemic is about 10 x 0.031 = 0.31 or 31%.
13 Berns KI1, Casadevall A, Cohen ML, Ehrlich SA, Enquist LW, Fitch JP, Franz DR, Fraser-Liggett CM, Grant CM, Imperiale MJ, Kanabrocki J, Keim PS, Lemon SM, Levy SB, Lumpkin JR, Miller JF, Murch R, Nance ME, Osterholm MT, Relman DA, Roth JA, Vidaver AK. Policy: Adaptations of avian flu virus are a cause for concern. Nature. 2012 Jan 31;482(7384):153-4. doi: 10.1038/482153a.
14 Berg P. The dual-use conundrum. Science. 2012 Sep 14;337(6100):1273. doi: 10.1126/science.1229789.
15 Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on Their Destruction. Signed at London, Moscow and Washington on 10 April 1972. Entered into force on 26 March 1975. Depositaries: UK, US and Soviet governments
16 Leitenberg M, Leonard J, Spertzel R. Biodefense crossing the line. Politics Life Sci. 2003 Sep;22(2):2-3. Epub 2004 May 17. https://www.cambridge.org/core/journals ... D2AF95A329
17 Tarini G. Keeping the Biological Weapons Convention relevant. Bulletin of the Atomic Scientists, 1 November 2016
18 Lentzos F and Koblentz GD. It’s time to modernize the bioweapons convention. Bulletin of the Atomic Scientists, 4 November 2016
19 Koblentz G and Lentzos F. 21st century biodefence: Risks, trade-offs & responsible science. ILPI Weapons of Mass Destruction Project, International Law and Policy Institute
20 Beerli C. Biological Weapons Review Conference: ICRC statement. International Committee of the Red Cross, 08 November 2016
21 Meier O, Governance or Arms Control? The Future of the Biological and Toxin Weapons Convention. Global Memo, Council of Councils Oct 26, 2016
Are lab-created potential pandemic pathogens biological weapons?
The possibility has been raised that matHPAIs could be used as biological weapons.
For instance, in a 2012 Comment in the science journal Nature13, the NSABB voiced their concern:
“Dual use is defined as research that could be used for good or bad purposes. We are now confronted by a potent, real-world example…If influenza A/H5N1 virus acquired the capacity for human-to-human spread and retained its current virulence, we could face an epidemic of significant proportions…Recently, several scientific research teams have achieved some success in modifying influenza A/H5N1 viruses such that they are now transmitted efficiently between mammals, in one instance with maintenance of high pathogenicity…these scientific results also represent a grave concern for global biosecurity, biosafety and public health. Could this knowledge, in the hands of malevolent individuals, organizations or governments, allow construction of a genetically altered influenza virus capable of causing a pandemic? ...Our concern is that publishing these experiments in detail would provide information to some person, organization or government that would help them to develop similar mammal-adapted influenza A/H5N1 viruses for harmful purposes.”
Another concerned voice is found in a lead editorial in the journal Science14 by Nobel Laureate Paul Berg:
“Recent research with a highly pathogenic influenza virus has highlighted the importance of this issue. Reviews of the influenza research concluded that given “the risk of accidental or malicious release,” the benefits of such studies must be well justified. Thus, specific guidelines must be enforced to thwart not only intentionally harmful outcomes but accidental releases as well… Earlier this year, the NSABB was embroiled in a high-profile decision regarding the publication of research on enhanced transmissibility of the avian H5N1 influenza virus. The principal concern was that publishing such findings might embolden those with sinister motives to use that information to create a worldwide pandemic.”
The Dual-Use Conundrum
by Paul Berg
Science
Vol. 337, Issue 6100, pp. 1273
DOI: 10.1126/science.1229789
September 14, 2012
Paul Berg is the Cahill Professor of Biochemistry, Emeritus, at the Stanford University School of Medicine, Palo Alto, CA. He received the Nobel Prize in Chemistry in 1980 and was an organizer of the Asilomar conference on recombinant DNA in 1975. Email: [email protected].
Scientists are increasingly able to create genetically modified microorganisms whose properties are perceived as being beneficial as well as potentially useful for malevolent purposes. In 2004, a committee of the U.S. National Academy of Sciences adopted the term “dual use” for instances in which genetic or biosynthetic manipulations create new microorganisms, which, although valuable scientifically, are susceptible to misuse.1 The premise was that the prospects for malevolent outcomes derive from deliberate actions to inflict specific or widespread harm. But in those and subsequent discussions, too little attention was given to the likelihood of an accidental laboratory release of modified agents that would allow them to spread in susceptible human populations. Recent research with a highly pathogenic influenza virus has highlighted the importance of this issue. Reviews of the influenza research concluded that given “the risk of accidental or malicious release,” the benefits of such studies must be well justified.2 Thus, specific guidelines must be enforced to thwart not only intentionally harmful outcomes but accidental releases as well.
The 2004 committee recommended that individual scientists and the editors of scientific journals exercise responsible judgment in undertaking and publishing experiments that describe the creation of, or could lead to, such biological agents of concern. This led to the creation of the U.S. National Science Advisory Board for Biosecurity (NSABB) to advise the federal government on policies governing publication, public communication, and dissemination of dual-use research methodologies and results. Recognizing that identifying all experiments or products of potential misuse was problematic, the NSABB adopted the term “dual-use research of concern” to focus on those entities that could, if misused, cause grave harm on a large scale.
Earlier this year, the NSABB was embroiled in a high-profile decision regarding the publication of research on enhanced transmissibility of the avian H5N1 influenza virus. The principal concern was that publishing such findings might embolden those with sinister motives to use that information to create a worldwide pandemic. The final outcome was to publish the papers in their entirety, reflecting a judgment that the risk of malicious actions was less than the benefits investigators would derive from an enhanced capability to design protective measures.3 Throughout that debate, far less attention was given to the probabilities of inadvertent release of the modified viral strains and the consequences for susceptible human populations. Past experience provides a useful lesson. In the 1970s, accidental release was at the center of the biosafety debate generated by the advent of recombinant DNA technology. A meeting of scientists at Asilomar in 1975 resulted in federal and institutional oversight and regulation of recombinant DNA research. In that case, specific modes of physical and biological containment, commensurate with the anticipated potential risks, were mandated to minimize the potential harm from an inadvertent release of genetically modified agents. There were also experiments that were forbidden for this reason. At that time, the possibility of state-initiated terrorism was considered but judged to be of lesser concern than accidents.
Although deliberate misuse might well pose a greater risk today, recent calculations suggest that in research with highly transmissible and virulent biological agents, accidental release remains of great concern.4 It is imperative, therefore, that funding agencies and research institutions pay special heed to the effectiveness of the containment being used to minimize the likelihood of either accidental or malicious release. It is also essential that all laboratory personnel be highly trained and tightly supervised to ensure biosecurity.
_______________
Notes:
1. National Research Council, Biotechnology Research in an Age of Terrorism (National Academy Press, Washington, DC, 2004).
2. M. Lipsitch et al., Science 336, 1529 (2012).
3. M. Enserink, Science 336, 1494 (2012).
4. L. C. Klotz, E. J. Sylvester, Bulletin of the Atomic Scientists (7 August 2012); http://thebulletin.org/web-edition/feat ... e-pandemic.
The phrases “malevolent individuals, organizations or governments,” “intentionally harmful outcomes,” and “sinister motives” describe employment of these lab-created pathogens as biological weapons.
The Biological Weapons Convention15 was written with a focus on military tactical biological weapons, where significant quantities would usually be employed. Article I of the convention speaks to this focus:
“Article I
Each State Party to this Convention undertakes never in any circumstances to develop, produce, stockpile or otherwise acquire or retain:
(1) Microbial or other biological agents, or toxins whatever their origin or method of production, of types and in quantities that have no justification for prophylactic, protective or other peaceful purposes;
(2) Weapons, equipment or means of delivery designed to use such agents or toxins for hostile purposes or in armed conflict.”
For lab-created PPPs, any quantity, however small, could seed an outbreak or pandemic. In this circumstance, development also implies production and stockpiling, since a single vial and one to a few infected individuals is all that is necessary to launch an attack.
From a military tactical point of view, however, lab-created PPPs would not be good biological weapons as they would boomerang back on the attackers, since they are highly transmissible. Nonetheless, a suicidal terrorist group or a desperate State might employ them as a last resort, or threaten to employ them as a means of extortion.
Call for action from the Parties to the BWC
When Fouchier and Kawaoka carried out their research, it was unlikely that biological weapons even crossed their minds. Since that possibility has now been brought up, researchers who are creating PPPs must take into account the biological weapons risk of dual use information and laboratory release of their pathogens. If there is little public-health benefit or little defense rationale for particular research, the Parties to the BWC should question whether it is biological weapons development and act accordingly.
This is a complex issue. The question of what constitutes biological weapons development is complicated. Many biodefense activities of the U.S Department of Homeland Security’s proposed and now abandoned National Biodefense Analysis and Countermeasures Center would be considered biological weapons development. As pointed out in a letter16 in the journal Politics and the Life Sciences: "Taken together, many of the [proposed] activities…— most particularly the ‘‘Store, Stabilize, Package, Disperse’’ sequence and the ‘‘Computational modeling of feasibility, methods, and scale of production’’ item — may constitute development in the guise of threat assessment, and they certainly will be interpreted that way."
Biodefense crossing the line
by Milton Leitenberg, Senior Research Scholar, Center for International and Security Studies at Maryland School of Public Policy, University of Maryland, College Park, MD, [email protected]
Ambassador James Leonard, Head of the United States Delegation to the Biological Weapons Convention Negotiations, 1972
Dr. Richard Spertzel, Former Deputy Director, USAMRIID, and Senior Biologist on the Staff of the United Nations Special Commission (UNSCOM), 1994-1998
September, 2003
© Association for Politics and the Life Sciences
Last February, on Monday the ninth, Lieutenant Colonel George W. Korch, Jr, Ph.D., United States Army, speaking in his capacity as Deputy Director of the National Biodefense Analysis and Countermeasures Center (NBACC), Fort Detrick, Maryland, addressed the 2004 Department of Defense Pest Management Workshop, meeting in Florida at the Jacksonville Naval Air Station. He spoke in the Main Ballroom of the River Cove Officers’ Club. As of this writing the workshop’s full schedule1 still shows a hypertext link to his remarks, but the link is no longer active. While it was active, as late as April, a copy of his remarks, presented as computer slides, could be downloaded to any computer, anywhere. It can still be found, unofficially.2
NBACC is to contain four separate centers, buildings for each of which are being built adjacent to the US Army Medical Research Institute of Infectious Diseases (USAMRIID). One of the four centers, the Biothreat Characterization Center, according to Dr. Korch’s slides, will carry out studies in some sixteen different subject areas, among which will be these:
• genetic engineering;
• susceptibility to current therapeutics;
• host-range studies;
• environmental stability;
• aerosol animal-model development;
• aerosol dynamics;
• novel packaging;
• novel delivery of threat;
• bioregulators and immunomodulators; and
• “Red Teaming,” which is to say duplication of threat scenarios.
Task areas for biothreat-agent (BTA) analysis and technical-threat assessment were summarized as “Acquire, Grow, Modify, Store, Stabilize, Package, Disperse.” Classical, emerging, and genetically engineered pathogens are to be characterized for their BTA potential. Aerobiology, aerosol physics, and environmental stability will be studied in wet-laboratory and computer-laboratory settings. “Computational modeling of feasibility, methods, and scale of production” will be undertaken, and “Red Team” operational scenarios and capabilities will be assessed. BTA use and countermeasure effectiveness will be studied “across the spectrum of potential attack scenarios” through “[h]igh-fidelity modeling and simulation.” And so forth.3
The rapidity of elaboration of American biodefense programs, their ambition and administrative aggressiveness, and the degree to which they push against the prohibitions of the Biological Weapons Convention (BWC), are startling.
The production and stockpiling of biological-weapons agents are not the only criteria by which an offensive biological weapons (BW) program is defined. They are only such a program’s most obvious terminal expressions. Taken together, many of the activities detailed above — most particularly the “Store, Stabilize, Package, Disperse” sequence and the “Computational modeling of feasibility, methods, and scale of production” item — may constitute development in the guise of threat assessment, and they certainly will be interpreted that way. Development is prohibited by the Biological Weapons Convention.
How would these activities differ from their counterparts in the pre-1969 US BW program except for production and stockpiling this time not being envisioned?4 In recent remarks elsewhere, Dr. Korch noted that one NBACC objective, creation of genetically engineered agents, might raise BWC compliance questions. Yet other NBACC objectives could prove even more problematic.
On April 28, 2004, at the conclusion of a year’s review, the Bush administration disclosed details of the new National Biodefense Directive.5 Among them, reportedly, was that “the US intelligence community is under orders to carry out studies examining the types of genetically engineered ‘bugs’ terrorists could be working on to mount an attack.”6 Surely, the “intelligence community” is the least appropriate place in the US government to “carry out” such work — and the most likely to lack adequate oversight. And does a program of this design bear any relation to the realistic level of threat presented by non-state actor “bioterrorists”?7 Recently declassified documents demonstrate that the US intelligence community possesses evidence demonstrating that interested terrorist groups — al Qaeda among them — still have no capability to work with classical BW agents and certainly cannot engineer agents genetically.
What will be the effect of NBACC’s work program on the worldwide evolution of BW over the next twenty or thirty years? Work on bioregulators and immunomodulators in the former Soviet offensive BW program during the 1980s is in retrospect realized to have been among the most dangerous and reprehensible of its numerous nefarious activities, despite having never approached weaponization, staying “safely” at research-and-development stages. Other than context — a preposterously huge offensive BW program — was work on bioregulators and immunomodulators qualitatively different from the work now to be carried out in the United States?
Will all the work in the categories listed above be classified, carried out under conditions of secrecy, or will it be open, generating peer-reviewed publications? The present US administration, if it was willing to scuttle attempts to finalize a verification protocol for the Biological Weapons Convention so as to shield the US biodefense program as constituted around 2001 and 2002, seems unlikely to welcome or even tolerate scrutiny of a program orders of magnitude larger and much closer to treaty breach. Yet circumstances are confounded still further. Some argue that a biodefense program can be legitimate only if it is transparent. That may be so, but if results to be sought as above were openly to be published then information plausibly facilitating BW efforts elsewhere would be disseminated.
Alternatively, if the US program proceeds in secret, what will be the reaction of other countries — including Russia and China? Will the twelve-year American-British effort to open major BW-capable facilities of the Russian Ministry of Defense be made more likely or less likely to succeed? Finally, will rivals steer currently legitimate biodefense programs down the new American path, but even more deeply in shadow?
References
1. < http://www.afpmb.org/pubs/misc/TriServi ... s-2004.pdf >.
2. < http://www.cbwtransparency.org/archive/nbacc.pdf >.
3. George Korch, “Leading Edge of Biodefense: The National Biodefense Analysis and Countermeasures Center,” Proceedings, Military Entomology — Its Global Challenge, 2004 DoD Pest Management Workshop, Naval Air Station, Jacksonville, Florida, February 9-13, 2004. Sponsored by the Armed Forces Pest Management Board Office of the Deputy Under Secretary of Defense (Installations and Environment), Washington, D.C.
4. Milton Leitenberg, “Distinguishing offensive from defensive biological weapons research,” Critical Reviews in Microbiology 29:3 (2003), pp. 223-257.
5. “Biodefense for the 21st Century,” Office of the President, April 2004; “HHS Fact Sheet. Biodefense Preparedness. Public Health Emergency Preparedness. Transforming America’s Capacity to Respond,” News Release, US Department of Health and Human Services, April 28, 2004; “Fact Sheet: Bush Signs Biodefense Presidential Directive,” US Department of State, April 28, 2004.
6. John Mintz, “Bioterrorism Procedures Are Outlined. Bush Directive Specifies Agency Responsibilities,” Washington Post, April 29, 2004
7. Milton Leitenberg, “Biological weapons and bioterrorism in the first years of the twenty-first century,” Politics and the Life Sciences, September 2002, 21:2, pp. 3-27.
Recent articles directed to the Eighth BWC Review Conference (for instance, see here,...
Keeping the Biological Weapons Convention relevant
by Gabrielle Tarini
Bulletin of the Atomic Scientists
November 1, 2016
Officials gathering in Geneva next week to examine the status of the Biological Weapons Convention (BWC) will have a choice between plodding along with the current, broken process or taking concrete steps to reinvigorate a treaty that is integral to the international security landscape. For the 41-year-old treaty, the upcoming Eighth Review Conference is a pivotal opportunity for countries to take action to ensure that the treaty remains a relevant and useful tool for preventing the development, spread, and use of biological weapons. A failure by member states to invest the necessary attention, time, and political capital in the conference could mean decreased interest and weakened multilateral engagement in a treaty that was the first to ban an entire category of weapons of mass destruction.
The treaty prohibits the possession of biological and toxin weapons. It covers a broad range limited primarily by intent: Parties to the Biological Weapons Convention agree not to develop, acquire, or retain agents, toxins, or delivery systems for non-peaceful purposes. The treaty has been tremendously successful in building a broad agreement that the life sciences should only be used for benign purposes, and a robust norm against the use of disease as a means of warfare. While membership in the BWC is not yet universal, no state claims that biological weapons are a legitimate means of national defense. Even countries that are thought to be pursuing biological weapons, such as North Korea, do not assert that they have a right to these weapons, or that biological weapons are a legitimate means of strategic deterrence.
The parties to the BWC agree that it is an important disarmament treaty representing a strong norm against biological weapons, but that is one of their few areas of agreement. Translating consensus into action has been difficult; the language adopted at previous Review Conferences has often repeated broad generalities and failed to advance a common agenda. This time around, the countries that are parties to the treaty should aim for fresh language and delineate specific actions to take.
Keeping pace with biotechnology.
The purpose of the Review Conference, held every five years, is to review the operation of the treaty and consider whether any new scientific and technological developments could enable activities that are inconsistent with the aims and objectives of the treaty, and that are not already covered by its provisions. There are several key issues at stake in the upcoming conference, scheduled for November 7–25.
Perhaps most critically, the BWC must find a more effective way to adapt to the rapid pace of scientific and technological change. Biotechnology methods and equipment are more powerful than ever, and barriers to their acquisition and use have eroded. For example, new gene-editing methods, such as Crispr, have significant biosecurity implications. Crispr has grabbed national headlines as the latest example of the dangers of dual-use technology. Earlier this year, Director of National Intelligence James R. Clapper named genome editing as a development with potential implications for the development of weapons of mass destruction, alongside North Korea’s nuclear weapons, new Russian cruise missiles, and undeclared chemical weapons in Syria.
Crispr is currently the most popular gene-editing method and has been revolutionizing scientific research. It is a unique technology that enables geneticists and medical researchers to edit parts of the genome by cutting out, replacing, or adding snippets to the DNA sequence. While genome editing itself is not a new process, older techniques are more difficult, less accurate, and quite expensive. The Crispr system is faster, more reliable, and cheaper. (The basic ingredients can be bought online for approximately $60.) The low cost and increased availability of these techniques have policymakers concerned that they could be used by individuals or groups with limited expertise and a lack of knowledge of safety and security precautions—or, even worse, by sub-state groups seeking to produce an enhanced pathogen to inflict harm on civilian populations.
Given the speed at which science and biotechnology are advancing, more effective arrangements are needed to present, digest, and discuss relevant developments—including Crispr and others—and their implications for the BWC. There are already inherent challenges in meaningfully addressing science and technology in a diplomatic meeting, and the current process only exacerbates these difficulties rather than providing effective workarounds.
Incorporating expert input.
Other international agreements, such as the Chemical Weapons Convention, have permanent advisory boards to track and respond to scientific change; the BWC, however, does not have a dedicated process to inform and advise member states. The Review Conference only occurs once every five years, so it cannot ensure timely consideration of scientific advances. Furthermore, the Review Conference must accomplish a myriad of other objectives, leaving insufficient meeting time to do justice to science and technology issues.
The most recent intersessional process added a Standing Agenda Item on developments in science and technology to the BWC’s annual Meeting of Experts, which has meant that, at the very least, treaty members will discuss relevant developments once a year. But even at the experts’ meeting, the latest developments are still getting lost in the general work of the BWC, and there is no opportunity for the experts’ conversations to be fed back into the policy process. What’s more, many countries do not show up to the Meeting of Experts, so they remain uninformed about new developments—and potential policies to deal with them. Treaty members should take action at the Review Conference to replace the current ad hoc process with a separate, structured, expert-led regime that will allow for the continuous monitoring and evaluation of developments in science and technology relevant to the BWC.
A stronger framework.
The Eighth Review Conference not only provides an opportunity to establish a stronger, more strategic scientific review process, but also offers a platform to revamp the intersessional process and institutional structures more broadly. Again, this is important because review conferences are not frequent enough to accomplish the laundry list of important objectives. Treaty members will have to think about new intersessional meetings, what format they should take, and which topics they should cover.
The countries that are part of the BWC will also have to consider the future of the Implementation Support Unit, because its mandate will expire next year. That unit is tasked with enormous responsibilities that far exceed the capabilities of its three-person staff: helping nations implement the treaty, providing support and assistance for confidence-building measures, administering a database of assistance requests and offers, and facilitating exchanges of information, to name just a few of its duties. It is high time for the Implementation Support Unit to be expanded.
The way in which discussions are planned and held should be restructured, with a stronger steering body and increased time for preparation and multilateral engagement. Adding more meetings, and limiting what gets discussed at each of those meetings, would allow the BWC to begin operating more like an international organization and would provide oversight equivalent to that for other nonproliferation treaties.
While the norm embodied in the BWC remains strong, the international community must go beyond raising awareness and toward more specific understandings about what countries should do to enhance the strength and influence of the treaty. Establishing a more strategic science and technology advisory process and strengthening the intersessional process and institutional structures are sound places to start.
Editor's note: A correction was made to this article to clarify that the treaty itself will not be restructured, but rather that the way in which treaty discussions are planned and held should be restructured.
here,...
It’s time to modernize the bioweapons convention
by Gregory D. Koblentz, Filippa Lentzos
Bulletin of the Atomic Scientists
November 4, 2016
On November 7, the Eighth Review Conference of the Biological Weapons Convention will commence in Geneva. Convened every five years, these meetings are an important opportunity to take stock of the treaty and its contribution to the global biosecurity regime.
The bioweapons convention is the cornerstone of the bioweapons nonproliferation regime. Together with the 1925 Geneva Protocol, it upholds a complete ban on the development, production, and use of biological weapons. The norm against these weapons is exceptionally strong. No state openly admits to pursuing a biological weapons capacity, and membership in the treaty continues to grow. Yet while the convention is not failing, it is not flourishing either. It lacks a dedicated forum to assess treaty implications of scientific advances, a robust institutional capacity, organized means of helping member nations meet their obligations, provisions for verifying compliance, and an operational role to respond in cases of a serious violations. The upcoming review conference provides a welcome opportunity to begin rectifying some of these shortcomings.
The risk of irrelevance.
The review of science and technology has been a standing agenda item of the treaty’s intersessional meetings over the last four years. While this sustained focus was a marked improvement, the overall experience has been disappointingly uneven. Despite rapid scientific and technological advances that have lowered the “barriers to acquiring and using a biological weapon,” the bioweapons convention has been unable to provide a forum where crucial contemporary debates about new developments—including gain-of-function experiments, potential pandemic pathogens, Crispr and other genome editing technologies, gene drives, and synthetic biology—can take place internationally. Many organizations and governments recognize the need for reform. The InterAcademy Partnership of national science academies has been particularly active in developing ideas and facilitating discussions on science and technology review, and a number of states, including Finland, Norway, Sweden, Spain, Switzerland, Russia, the United Kingdom, and the United States, have put forward concrete suggestions for how to improve the review process, but major disagreement remains on the purpose, structure, membership, and funding of the science advisory group.
And it is not just relevant scientific debates that are taking place elsewhere. The center of efforts to prevent biological terrorism and the spread of bioweapons is starting to shift away from the convention toward UN Security Council Resolution 1540. First approved in 2004, this measure imposes an obligation on all UN members to improve their legal authorities and bureaucratic capacities to prevent non-state actors from acquiring, developing, or using nuclear, biological, and chemical weapons. While the bioweapons treaty provides one of the foundations for 1540’s mandate, the resolution appears to be becoming the preferred international vehicle for enhancing biosafety, biosecurity, export controls, and the criminalization of biological weapons. This situation is due in large part to the stronger political backing and larger administrative capacity devoted to the implementation of 1540. While the convention’s administrative body, the Implementation Support Unit, has a staff of three that reports to the UN Office of Disarmament Affairs, the 1540 Committee is composed of diplomats from 18 nations, is supported by a group of nine experts on loan from their home countries, and reports to the Security Council. While the overall bioweapons nonproliferation regime has benefited greatly from 1540, without a major influx of resources to the treaty’s implementation unit, the original mission of the regime risks becoming relegated to better-funded organizations.
Such a shift would also have ramifications for the way in which the convention reaches out to stakeholders—from scientists and science academies, health professionals and first responders, to members of humanitarian organizations, academia, and the private sector—and incorporates them into discussions about biological risk management. Because the 1540 Committee is focused primarily on nuclear proliferation, it concentrates on reducing material- and equipment-based threats, privileges legal tools such as criminalization and export controls, and engages with stakeholders at arm’s length. While that approach makes sense for the 1540 Committee, given the resolution’s origin as a response to the September 11 terrorist attacks and revelations about the A. Q. Khan nuclear proliferation network, it is not comprehensive enough for the biological field, where arms control and nonproliferation efforts are primarily about the ongoing management of a knowledge-based risk. Hence the need for the bioweapons convention to regain its rightful place as the premier international forum for countering biological terrorism and proliferation, a need that can best be met by giving the treaty’s implementation unit more funding and resources.
Finally, the review conference should also address the issue of transparency, which is an important tool for reassuring members of one another’s compliance with treaty obligations. The last two decades have seen a dramatic increase in biodefense activities and the number of facilities and researchers working with dangerous pathogens around the world. This has generated a number of trade-off risks related to safety, security, responsible science, and particularly transparency. A major risk here is that these expanding activities could be used as a cover for an offensive bioweapons program, or could be perceived as such. This, in turn, can provide other states with a justification for initiating or continuing offensive biowarfare programs. Only by encouraging trust and transparency among its members can the treaty hope to prevent such an escalation.
Making reforms work.
In anticipation of the Eighth Review Conference, the Biodefense Graduate Program at the Schar School of Policy and Government, at George Mason University, and the Department of Global Health & Social Medicine, at King’s College London, convened a small roundtable of academics, policy makers, and former government officials from the United States and the United Kingdom to consider the state of the bioweapons convention and the challenges it is facing. Three strong themes, discussed above, emerged from the talks: adapting to advances in biology and the life sciences, countering treaty marginalization, and increasing the transparency of biodefense programs. It is imperative for the review conference to take concrete steps to address these shortcomings.
First, members should decide to organize the review of relevant developments in science and technology more systematically, and resource it more fully, through an Open-Ended Working Group on Science and Technology supported by additional dedicated staff assigned to the Implementation Support Unit. The working group should be composed of experts nominated by governments and supplemented by specialists from academia, civil society, and industry, and it should regularly review advances in science and technology and assess potential impacts (both positive and negative) on the objectives of the bioweapons convention. The rapid pace of scientific innovation and unexpected breakthroughs, such as the recent discovery of the gene-editing tool Crispr, argue for a flexible approach that can draw on a wide range of expertise as needed. The working group should also be empowered to produce recommendations to member states.
Second, the review conference needs to renew the mandate of its implementation unit, and more importantly provide it desperately needed resources to expand its size and give it a realistic operating budget. Five years ago the Seventh Review Conference added new tasks to the unit but, at the last minute, refused to pay for them. As a result, the unit has had to draw attention, in each of its annual reports since 2012, to the work it has not been able to do, for lack of resources. Increasing the staff of the unit by two positions, including staff dedicated to overseeing the science and technology process, is a reasonable proposition. A better budget would still only ask treaty members to contribute less than 5 percent of what member nations are required to give the Organization for the Prohibition of Chemical Weapons, the inspection agency of the Chemical Weapons Convention. Even allowing for major differences between the two conventions in terms of institutional capacity and international verification activities, this is disproportionate. Running the bioweapons convention on an inadequate budget sends a terrible message about how seriously its members regard the treaty as part the bioweapons nonproliferation regime, not to mention how seriously they prioritize implementation.
Finally, treaty members should set up an Open-Ended Working Group on Providing Reassurance to encourage transparency and participation in “peer review” exercises to reassure one another that biodefense programs are in full compliance with the convention. Unusually for an arms control treaty, the bioweapons convention was agreed to without a system for routine on-site verification activities. Efforts to introduce a legally binding verification mechanism for the treaty have failed in the past, and developments in the political, security, and scientific contexts are making it increasingly clear that a fully effective verification system, or for that matter absolute certainty about the full compliance of treaty members, is exceptionally difficult.
Yet this does not mean that it is impossible for states to be assured that other nations are abiding by their treaty obligations. There are a number of actions and activities that cumulatively may give a reasonable indication of a member nation’s compliance status over time. Most states with biodefense programs recognize their special responsibility to ensure high standards of transparency. They submit declarations about their programs, as required under the treaty’s confidence-building measures, to reassure other states that their activities are solely for peaceful purposes. To maximize their transparency, an increasing number of states (as of this year, 18 of the 29 members with declared biodefense programs) are now also making their declarations publicly available. Recently, a small number of states, including France, Germany, Belgium, and the Netherlands, have voluntarily gone even further by inviting experts from other states, including non-government experts, to their biodefense facilities for interactive information exchanges and on-site visits. These innovative peer-review exercises are designed to provide reassurance through transparency and must be strongly endorsed at the Eighth Review Conference. It is through these sorts of initiatives that member nations can develop common understandings of how best to reassure one another, and the wider world, that their biodefense activities serve “prophylactic, protective and other peaceful purposes” and therefore are permitted by the treaty.
The Eighth Review Conference provides an opportunity to revitalize the bioweapons treaty by taking concrete actions to expand its relevance, enhance its capacity to review developments in science and technology, and strengthen the confidence of nations in the peaceful intentions of their fellow treaty members. These proposed measures will also enable the regime to play a leading role in the global dialogue on preventing the misuse of biology. The repeated use of chemical weapons by Syria and the Islamic State should serve as a stark reminder about the importance of ensuring that advances in the life sciences—which have the potential to create weapons exponentially more dangerous than chlorine barrel bombs—are not exploited by states or non-state actors for hostile purposes.
[url]here[/url], here,...
Biological Weapons Review Conference: ICRC statement
by Christine Beerli, vice-president of the ICRC
International Committee of the Red Cross
November 8, 2016
Eighth Review Conference of the States Parties to the Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on their Destruction, Statement by Christine Beerli, vice-president of the ICRC.Poisoning and the deliberate spread of disease are unacceptable in any circumstances, and we must do everything possible to ensure that the life processes at the core of human existence are never manipulated for hostile purposes.
Society has long rejected poisoning and the deliberate spread of disease as abhorrent means of warfare. The prohibition of the use of biological weapons -– embodied in the 1925 Geneva Protocol and the Biological and Toxin Weapons Convention (BWC) -– is a rule of customary international humanitarian law. It is binding on all parties to all armed conflicts, be they States or non-State armed groups. The ban is absolute and far-reaching, covering everything from the hostile use of biological agents by individuals or groups for criminal or terrorist ends, to the penal sanctions that States Parties are required to impose nationally.
As emphasized in the final documents of past Review Conferences, and especially in light of continuing scientific developments, the absolute prohibition on use of biological weapons ‒- encompassing all biological agents, whatever their origin -– must be reaffirmed by this Review Conference.
States Parties should not become complacent; it remains their collective and individual responsibility to ensure that the treaty is implemented effectively. Over the past five years of annual meetings, a great deal of information has been shared and many proposals have been made on how to implement the treaty and improve its effectiveness. Disappointingly, however, there has been little collective agreement.
The International Committee of the Red Cross (ICRC) urges States Parties to seize the opportunity of this Review Conference to agree on concrete and practical measures, including an effective programme of work for 2017 and beyond, to reduce the risks to life and health posed by biological weapons and, ultimately, protect humanity from the horrific effects of these weapons.
We have only to consider the devastating impact that outbreaks of diseases, such as Ebola, have had on public health, economic well-being, and national and international security to appreciate how important it is to prevent the deliberate, and even accidental, spread of disease.
Meanwhile, scientific and technological advances could make biological weapons cheaper to obtain, easier to use, deadlier in their effects and harder to detect. Scientists have pointed out that, in the five years since the last Review Conference, the technological barriers to developing and using biological weapons have been significantly lowered.
The ICRC proposes five concrete actions to strengthen the prohibition of biological weapons ‒- many of them contained in the working papers submitted by States Parties -‒ that should be taken as a result of this Review Conference.
First, States Parties should develop effective means to monitor and assess compliance with the BWC. Fifteen years after negotiations on a verification protocol failed, this fundamental issue deserves renewed attention. It is now time to explore the full range of ideas on and approaches to compliance monitoring. As a first step, the ICRC encourages this Review Conference to establish a working group -– or similar process –- to take this issue forward from 2017.
Second, States Parties must remain prepared to respond and assist each other in the event that biological weapons are used. Shared efforts to increase preparedness should focus on enhancing capabilities to assist victims of any such attack.
Practical support is therefore crucial to ensuring that the measures under Article VII on the provision of assistance are implemented. This Review Conference should establish a working group -– or similar process –- to agree on how to build up response capacity where it is lacking, improve coordination among those who may be involved, address current obstacles to providing an effective response and, ultimately, limit the repercussions in humanitarian terms of any use of biological weapons.
The ICRC has in the past drawn attention to the lack of international capacity to assist victims in the event biological weapons are used. The challenges made evident by the international humanitarian response to the outbreak of Ebola from 2014 to 2016 underscore the urgent need for progress in this area. Lessons learned from this natural outbreak can be used to improve the capacity to respond in a deliberate attack, as outlined in Working Paper 39, submitted by the ICRC to the August Preparatory Committee.
Third, the ICRC urges this Review Conference to establish an effective mechanism for assessing the implications of developments in science and technology for the BWC. States Parties must remain up to date with fast-moving scientific and technological developments and their potential risks in order to prevent the development and use of biological weapons while ensuring that biological research for peaceful and beneficial purposes remains unhindered.
Fourth, States Parties must continue their efforts to promote universal ratification or accession to the BWC. There is no reason why any State should not be party to the treaty. The ICRC welcomes the four new States Parties for 2016, Côte d'Ivoire, Angola, Liberia and Nepal and we urge all States that have not yet done so to ratify or accede to the BWC without delay. We also call on States still holding reservations to the Geneva Protocol to withdraw them.
Fifth, sustained effort is needed on effective domestic implementation of the treaty. Legally, as well as for public-health and security reasons, States Parties must ensure that their domestic laws reflect international obligations, and that appropriate biosafety, biosecurity, export-control and enforcement measures are in place. Because this is such a key area, the ICRC convened a meeting in early October of this year to facilitate sharing of best practices among government experts in the South Asia region.
Poisoning and the deliberate spread of disease are unacceptable in any circumstances, and we must do everything possible to ensure that the life processes at the core of human existence are never manipulated for hostile purposes.
In joining the BWC, States Parties have made a solemn commitment “for the sake of all mankind, to exclude the possibility of bacteriological (biological) agents and toxins being used as weapons”. The world will be watching closely to see whether this commitment will be translated into action.
and here) 17,18,19, 20,21 call for the Parties to intensify their focus on new science and technology that could lead to violations of the BWC. Lab-created PPPs, particularly matHPAI, because they are already present in laboratories around the world, are an urgent focus.
Article XII of the BWC calls for
“review [of] the operation of the Convention…assuring that the purposes of the preamble and the provisions of the Convention…are being realized. Such review shall take into account any new scientific and technological developments relevant to the Convention.”
Hopefully, the States Parties to the BWC will set in motion a process for overseeing relevant new research and technologies. If the Parties decide lab-created PPPs are within its mandate under Article XII of the BWC, it could speed up the enactment of guidelines and regulations. At the very least, the Parties should be the catalyst to launch discussions for a different international treaty on oversight and regulation of creation and research on highly dangerous agents. In the meantime, since enacting new treaties is an uncertain and long process, Parties to the BWC should pass legislation in their own nations.
_______________
Notes:
1 Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012 Jun 22;336(6088):1534-41. doi: 10.1126/science.1213362
2 Masaki Imai, Tokiko Watanabe, Masato Hatta, Subash C. Das, Makoto Ozawa, Kyoko Shinya, Gongxun Zhong, Anthony Hanson, Hiroaki Katsura, Shinji Watanabe, Chengjun Li, Eiryo Kawakami, Shinya Yamada, Maki Kiso, Yasuo Suzuki, Eileen A. Maher, Gabriele Neumann & Yoshihiro Kawaoka. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012 May 2;486(7403):420-8. doi: 10.1038/nature10831
3 Fouchier RA, Kawaoka Y, Cardona C, Compans RW, García-Sastre A, Govorkova EA, et al. Gain of-function experiments on H7N9. Science. 2013 Aug 9;341(6146):612-3. doi: 10.1126/science.341.6146.612
4 Fouchier RA, Kawaoka Y, Cardona C, Compans RW, Fouchier RA, García-Sastre A, et al. Avian flu: gain-of-function experiments on H7N9. Nature. 2013 Aug 8;500(7461):150-1. doi: 10.1038/500150a.
5 Klotz LC. The Potential Pandemic Influenza Research Enterprise. https://armscontrolcenter.org/wpcontent ... ersion.pdf
6 Panel on Scientific Communication and National Security. Committee on Science, Engineering, and Public Policy: National Academy of Sciences, National Academy of Engineering. Institute of Medicine. Scientific Communication and National Security, NATIONAL ACADEMY PRESS Washington, D.C. 1982
7 Relman DA. "Inconvenient truths" in the pursuit of scientific knowledge and public health. J Infect Dis. 2014 Jan 15;209(2):170-2. doi: 10.1093/infdis/jit529. Epub 2013 Oct 7.
8 Koblentz GD. The De Novo Synthesis of Horsepox Virus: Implications for Biosecurity and Recommendati for Preventing the Reemergence of Smallpox. Health Secur. 2017 Aug 24. doi: 10.1089/hs.2017.0061. [Epub ahead of print]
9 Klotz LC1, Sylvester EJ. The consequences of a lab escape of a potential pandemic pathogen. Front Public Health. 2014 Aug 11;2:116. doi: 10.3389/fpubh.2014.00116
10 Lipsitch M1, Cohen T, Cooper B, Robins JM, Ma S, James L, Gopalakrishna G, Chew SK, Tan CC, Samore MH, Fisman D, Murray M. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003 Jun 20;300(5627):1966-70. Epub 2003 May 23. See Figure 4a.
11 Merler S, Ajelli M, Fumanelli L, Vespignani A. Containing the accidental laboratory escape of potential pandemic influenza viruses. BMC Med. 2013 Nov 28;11:252. doi: 10.1186/1741-7015-11-252.
12 The yearly probability of a natural pandemic may be estimated as follows: The number of years that have passed since the beginning of 1920 until September 8, 2017 = 97.77 years. (The proper starting date is 1920, just after the first pandemic, the 1918 pandemic flu. Any other starting date would be arbitrary.) There have been three pandemics since 1920: 1957, 1968 and 2009. Thus, the probability of a pandemic in any one year is (3 pandemics) / (97.7 years) = 0.031 per year. In ten years, the probability of a natural pandemic is about 10 x 0.031 = 0.31 or 31%.
13 Berns KI1, Casadevall A, Cohen ML, Ehrlich SA, Enquist LW, Fitch JP, Franz DR, Fraser-Liggett CM, Grant CM, Imperiale MJ, Kanabrocki J, Keim PS, Lemon SM, Levy SB, Lumpkin JR, Miller JF, Murch R, Nance ME, Osterholm MT, Relman DA, Roth JA, Vidaver AK. Policy: Adaptations of avian flu virus are a cause for concern. Nature. 2012 Jan 31;482(7384):153-4. doi: 10.1038/482153a.
14 Berg P. The dual-use conundrum. Science. 2012 Sep 14;337(6100):1273. doi: 10.1126/science.1229789.
15 Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on Their Destruction. Signed at London, Moscow and Washington on 10 April 1972. Entered into force on 26 March 1975. Depositaries: UK, US and Soviet governments
16 Leitenberg M, Leonard J, Spertzel R. Biodefense crossing the line. Politics Life Sci. 2003 Sep;22(2):2-3. Epub 2004 May 17. https://www.cambridge.org/core/journals ... D2AF95A329
17 Tarini G. Keeping the Biological Weapons Convention relevant. Bulletin of the Atomic Scientists, 1 November 2016
18 Lentzos F and Koblentz GD. It’s time to modernize the bioweapons convention. Bulletin of the Atomic Scientists, 4 November 2016
19 Koblentz G and Lentzos F. 21st century biodefence: Risks, trade-offs & responsible science. ILPI Weapons of Mass Destruction Project, International Law and Policy Institute
20 Beerli C. Biological Weapons Review Conference: ICRC statement. International Committee of the Red Cross, 08 November 2016
21 Meier O, Governance or Arms Control? The Future of the Biological and Toxin Weapons Convention. Global Memo, Council of Councils Oct 26, 2016
- admin
- Site Admin
- Posts: 40066
- Joined: Thu Aug 01, 2013 5:21 am
Re: U.S. government gave $3.7 million grant to Wuhan lab at
The consequences of a lab escape of a potential pandemic pathogen
by Lynn C. Klotz, The Center for Arms Control and Non-Proliferation, Washington, DC, USA; and Edward J. Sylvester, Science and Medical Journalism, Walter Cronkite School of Journalism and Mass Communication, Arizona State University, Phoenix, AZ, USA
Frontiers in Public Health
August 11, 2014
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT
In letters to the journals Science and Nature (1, 2), 22 virologists notified the research community of their interest in expanding research to develop strains of the already deadly H7N9 Asian influenza virus that would be transmissible via aerosols among mammals, thus creating potential pandemic pathogens. PPPs are defined as pathogens that are potentially highly contagious, potentially highly deadly, and not currently present in the human population. Mammalian contagious avian flu, the 1918 pandemic flu, and SARS are examples. The letter writers cite their scientific reasons for the need for such research, much the same reasons as given by those working on similar projects for the H5N1 avian flu virus (3, 4). This new proposed research signals wider interest in making dangerous influenza viruses (5, 6) contagious in mammals via respiratory aerosols. At present, there are no international regulations or guidelines in place to decide whether such a research project should proceed.
Now is the time to address the next critical question: what is the likelihood that one of these viruses will escape from a lab and seed the very pandemic the researchers claim they are trying to prevent? As we shall estimate, that probability could be as high as 27%, a risk too dangerous to live with.
First, from the calculations in two in-depth pandemic risk analyses (7–9), there is a substantial probability that a pandemic with over a 100-million fatalities could be seeded from an undetected lab-acquired infection (LAI), if a single infected lab worker spreads infection as he moves about in the community. From the Klotz (2014) analysis, there is about a 1–30% probability, depending on assumptions, that, once infected, the lab worker will seed a pandemic. This large probability spread arises from varying the average number of people infected by an infected person between 1.4 and 3.0 (R0, in standard epidemiology notation), varying the details of commutes to and from work on public transportation, and whether infected acquaintances are quarantined before spreading infection. The Merler (2013) study, based on a computer-generated population grid of size and varying density of the Netherlands, supports our concern over a lab escape not being detected until it is too late: “there is a non-negligible probability (5–15%), strongly dependent on reproduction number and probability of developing clinical symptoms, that the escape event is not detected at all.”
Different methodologies were used in the Klotz (2014) and Merler (2013) risk analyses. Additional analyses are needed using other methodologies, such as the mathematical model employed for SARS (10), which hopefully will lead to some consensus on risk. The Klotz and Merler studies, however, are the first to raise these concerns and point to valid issues about the potential risks from a single LAI.
Given such a dire predicted outcome by the existing studies, the critical question is: what is the probability that a worker acquires an undetected infection in the lab in the first place? To answer this question, we reproduce here one part of the Klotz (2014) analysis: the probability of an escape through an LAI from at least one of the many labs expected to be involved in this research enterprise.
A 2013 Centers for Disease Control report is a significant source of recent data on LAIs (11). The report documents four undetected or unreported LAIs in registered US Select Agent, high-containment BSL-3 labs between 2004 and 2010. An undetected or unreported LAI implies an escape when the infected person leaves the lab. The report identifies an average of 292 registered Select Agent BSL-2, BSL-3, and BSL-4 labs operating over those 7 years, for a total of 292 × 7 = 2,044 lab years. Unfortunately, the study does not break down numbers into BSL-2, BSL-3, and BSL-4 labs or lab years.
Thus, the probability of escape for a single year, p1, can only be calculated as 4 LAIs/2,044 lab years = 0.002 or 0.2% per lab per year. This is clearly an underestimate since BSL-2 and BSL-4 labs contribute to the denominator. (The denominator used here, 2,004, equals the number of BSL-2 plus number of BSL-3 plus number of BSL-4 labs. But the denominator in our calculation should be just the number of BSL-3 labs, so the denominator is overestimated and the percent escape is then underestimated. Although requested, the CDC has not supplied us with the number of BSL-3 labs for us to do the exact calculation.) This basic probability is consistent with that for SARS escapes in Asia through LAIs (12) and with all known escapes from BSL-4 labs in the Soviet Union from LAIs and Great Britain from a mechanical failure (13).
To illustrate potential risk, the probability of no escape from a single lab in a single year is (1 − p1), so
is the probability of no escape from N labs in Y years. And
is the probability of at least one escape from N labs in Y years.
Given the Science and Nature articles listed above (1, 2), it is reasonable to assume that at least 10 labs will undertake this research and that this work would continue for 10 years, so
or an 18% likelihood of at least one escape from at least one lab for the whole research enterprise, almost 100-times greater than the likelihood for a single lab in a single year.
We noted above that the probability p1 = 0.2% is conservative, estimated from the CDC data alone. The first Department of Homeland Security risk assessment for the planned National Bio- and Agro-Defense Facility in Manhattan, Kansas estimated a significantly higher escape risk, over 70% likelihood for the 50-year life of the facility (14), which works out to be a basic probability of escape, p1 = 2.4% per year. The National Research Council (14) overseeing the risk assessment remarked “The … estimates indicate that the probability of an infection resulting from a laboratory release of FMDv from the NBAF in Manhattan, Kansas approaches 70% over 50 years (see Figure 3-1) with an economic impact of $9–50 billion. The committee finds that the risks and costs could well be significantly higher than that…” While the DHS subsequently lowered the escape risk to 0.11% for the 50-year lifetime (14), the NRC committee (14) was highly critical of the new calculations: “The committee finds that the extremely low probabilities of release are based on overly optimistic and unsupported estimates of human error rates, underestimates of infectious material available for release, and inappropriate treatment of dependencies, uncertainties, and sensitivities in calculating release probabilities.” We have more trust in the NRC committee conclusions, as they have no skin in the game.
With this higher number, which we take as a worst-case scenario, the likelihood of at least one escape from 10 labs in 10 years becomes 91%, almost a certainty. It follows that, if the likelihood of one LAI leading to a pandemic is 30% in the worst-case scenario, the likelihood of an LAI-caused pandemic resulting from this whole research enterprise could be as high as 30 × 91% = 27%, a likelihood that is too dangerous to live with, as we noted. While this represents a worst-case scenario, it is not improbable.
Recent self-reported mistakes at the CDC (15), involving a particularly deadly strain of anthrax removed from BSL-3 containment and H5N1 Asian bird flu released from the CDC laboratories altogether, lend support to our concern that the probability of escape may be much greater than the 0.2% per lab per year from just LAIs. The CDC report spawned a congressional inquiry (16) and led to dozens of newspaper articles with concerns about lack of safety in high-containment laboratories.
Our concern is shared by many virologists and epidemiologists. A recent letter to the President of the European Commission (17) co-signed by 56 scientists from more than a dozen countries warned, “The probabilities of a lab accident that leads to a global spread of an escaped mutated virus are small but finite, while the impact of global spread could be catastrophic.” The European Centre for Disease Prevention and Control (18) weighed-in with its concerns as well, as did the Cambridge Working Group (19). It must be noted that some of the signers of the European Commission letter and the Cambridge Working Group’s consensus statement are the same.
The risk of a man-made pandemic from a lab escape is not hypothetical. Lab escapes of high-consequence pathogens resulting in transmission beyond lab personnel have occurred (20, 21). The historical record reveals lab-originated outbreaks and deaths due to the causative agents of the 1977 pandemic flu, smallpox escapes in Great Britain, Venezuelan equine encephalitis in 1995, SARS outbreaks after the SARS epidemic, and foot and mouth disease in the UK in 2007. Ironically, these labs were working with pathogens to prevent the very outbreaks that they ultimately caused.
Do benefits outweigh risks? Those who support PPP experiments either believe the probability of PPP escape is infinitesimal or the benefits in preventing a pandemic are great enough to justify the risk. In making decisions for what lines of research will lead to new knowledge, experts must rely on intuition honed by years of research in a particular field. In the case of this PPP research, in our opinion it would take extraordinary benefits and significant reduction of risk via extraordinary biosafety measures to correct such a massive overbalance of highly uncertain benefits to too-likely risks (Wain-Hobson, 2013).
Whatever number we are gambling with, it is clearly far too high a risk to human lives. This Asian bird flu virus research to develop strains transmissible via aerosols among mammals, and perhaps some other PPP research as well, should for the present be banned. We must emphasize that we have been considering only a very small subset of pathogen research. Most pathogen research should proceed unimpeded by unnecessary regulations.
Special precautions in BSL-4 laboratories for work with PPPs should be adopted (22). These would include:
• Training a full-time technical staff for work with PPPs. Experiments could be directed by scientists outside the laboratory using modern audio-video technology.
• Requiring the staff to follow up extended work shifts with periods of quarantine before they leave the containment area to assure that no PPP escapes from the containment area through an LAI.
• Restricting these PPP laboratories to remote locations, where an aerosol escape or other containment failure would pose the least risk of infecting an outside community.
We label BSL-4 laboratories with the special precautions, BSL-4+. While PPP experiments would be carried out primarily under BSL-4+ containment, BSL-3 containment with the special precautions might suffice for some work.
Given the global threat, the international community should insist on discussions leading to an international agreement that would require the strictest oversight to conduct this particular research anywhere. To place responsibility with the international community where it belongs and to provide maximum transparency, policy makers should require that international inspectors have access to facilities at any time on short notice.
As it stands, there is no proactive oversight nor regulations for this PPP research, so any and all of the world’s nations can carry out this dangerous work without regard to consequences. But consequences would be shared by all of us. In the meantime, insurance companies who routinely provide insurance for biological research should consider excluding such risky research from coverage.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Fouchier RA, Kawaoka Y, Cardona C, Compans RW, García-Sastre A, Govorkova EA, et al. Gain-of-function experiments on H7N9. Science (2013) 341:612–3. doi: 10.1126/science.1243325
2. Fouchier RA, Kawaoka Y, Cardona C, Compans RW, Fouchier RA, García-Sastre A, et al. Avian flu: gain-of-function experiments on H7N9. Nature (2013) 500:150–1. doi:10.1038/500150a
3. Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science (2012) 336:1534–41. doi:10.1126/science.1213362
4. Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature (2012) 486:420–8. doi:10.1038/nature10831
5. Zhang Y, Zhang Q, Kong H, Jiang Y, Gao Y, Deng G, et al. H5N1 hybrid viruses bearing 2009/H1N1 virus genes transmit in guinea pigs by respiratory droplet. Science (2013) 340:1459–63. doi:10.1126/science.1229455
6. Sutton TC, Finch C, Shao H, Angel M, Chen H, Capua I, et al. Airborne transmission of highly pathogenic H7N1 influenza in ferrets. J Virol (2014) 88:6623–35. doi:10.1128/JVI.02765-13
7. Klotz LC. The Human Fatality and Economic Burden of a Man-made Influenza Pandemic: A Risk Assessment. The Center for Arms Control & Non-Proliferation (2014). Available from: http://armscontrolcenter.org/The_Human_ ... 1-5-14.pdf or http://bio-security.org/wp-content/uplo ... 1-5-14.pdf
8. Klotz L, Sylvester S. Opinion: The Fatality Burden. (2013). Available from: http://www.the-scientist.com/?articles. ... ty-Burden/
9. Merler S, Ajelli M, Fumanelli L, Vespignani A. Containing the accidental lab escape of potential pandemic influenza viruses. BMC Med (2013) 11:252. doi:10.1186/1741-7015-11-252
10. Lipsitch M, Cohen T, Cooper B, Robins JM, Ma S, James L, et al. Transmission dynamics and control of severe acute respiratory syndrome. Science (2003) 300:1966–70. doi:10.1126/science.1086616
11. Henkel RD, Miller T, Weyant RS. Monitoring select agent theft, loss and release reports in the United States 2004-2010. Appl Biosafety (2012) 17:171–80. Available from: http://www.absa.org/abj/abj/121704FAHenkel.pdf
12. Specific Incidences of Lab-Acquired SARS-CoV Infections. Centers for Disease Control and Prevention (2005). Available from: http://www.cdc.gov/sars/lab/biosafety.html
13. Klotz LC. Sharpening Our Intuition on Man-made Pandemics. Breeding BioSecurity Blog (2012). Available from: http://bio-security.org/wp-content/uplo ... on0515.pdf
14. Evaluation of the Updated Site-Specific Risk Assessment for the National Bio- and Agro-Defense Facility in Manhattan, Kansas. The National Academies Press (2012). Available from: http://www.nap.edu/catalog.php?record_id=13418
15. Report on the Potential Exposure to Anthrax. Centers for Disease Control and Prevention (2014). Available from: http://www.cdc.gov/od/science/integrity ... Report.pdf
16. Review of CDC Anthrax Lab Incident. Energy & Commerce Committee, United States House of Representatives. (2014). Available from: http://energycommerce.house.gov/hearing ... dent#video
17. Wain-Hobson S. Response to Letter by the European Society for Virology on “Gain-of-Function” Influenza Research. The Foundation for Vaccine Research (2013). Available from: http://www.nature.com/polopoly_fs/7.145 ... letter.pdf
18. Circulating Avian Influenza Viruses Closely Related to the 1918 Virus Have Pandemic Potential. European Centre for Disease Prevention and Control. (2014). Available from: http://ecdc.europa.eu/en/activities/sci ... d72&ID=765
19. Cambridge Working Group Consensus Statement on the Creation of Potential Pandemic Pathogens (PPPs). Available from: http://www.cambridgeworkinggroup.org/
20. Furmanski M. Lab Escapes and “Self-fulfilling prophecy” Epidemics. Center for Arms Control and Nonproliferation (2014). Available from: http://armscontrolcenter.org/Escaped_Vi ... -17-14.pdf
21. Furmanski M. Threatened pandemics and lab escapes: self-fulfilling prophecies. Bull Atom Sci (2014). Available from: http://thebulletin.org/threatened-pande ... hecies7016
22. Klotz LC, Sylvester EJ. The unacceptable risks of a man-made pandemic. Bull Atom Sci (2012). Available from: http://thebulletin.org/unacceptable-ris ... e-pandemic
by Lynn C. Klotz, The Center for Arms Control and Non-Proliferation, Washington, DC, USA; and Edward J. Sylvester, Science and Medical Journalism, Walter Cronkite School of Journalism and Mass Communication, Arizona State University, Phoenix, AZ, USA
Frontiers in Public Health
August 11, 2014
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT
YOU ARE REQUIRED TO READ THE COPYRIGHT NOTICE AT THIS LINK BEFORE YOU READ THE FOLLOWING WORK, THAT IS AVAILABLE SOLELY FOR PRIVATE STUDY, SCHOLARSHIP OR RESEARCH PURSUANT TO 17 U.S.C. SECTION 107 AND 108. IN THE EVENT THAT THE LIBRARY DETERMINES THAT UNLAWFUL COPYING OF THIS WORK HAS OCCURRED, THE LIBRARY HAS THE RIGHT TO BLOCK THE I.P. ADDRESS AT WHICH THE UNLAWFUL COPYING APPEARED TO HAVE OCCURRED. THANK YOU FOR RESPECTING THE RIGHTS OF COPYRIGHT OWNERS.
In letters to the journals Science and Nature (1, 2), 22 virologists notified the research community of their interest in expanding research to develop strains of the already deadly H7N9 Asian influenza virus that would be transmissible via aerosols among mammals, thus creating potential pandemic pathogens. PPPs are defined as pathogens that are potentially highly contagious, potentially highly deadly, and not currently present in the human population. Mammalian contagious avian flu, the 1918 pandemic flu, and SARS are examples. The letter writers cite their scientific reasons for the need for such research, much the same reasons as given by those working on similar projects for the H5N1 avian flu virus (3, 4). This new proposed research signals wider interest in making dangerous influenza viruses (5, 6) contagious in mammals via respiratory aerosols. At present, there are no international regulations or guidelines in place to decide whether such a research project should proceed.
Now is the time to address the next critical question: what is the likelihood that one of these viruses will escape from a lab and seed the very pandemic the researchers claim they are trying to prevent? As we shall estimate, that probability could be as high as 27%, a risk too dangerous to live with.
First, from the calculations in two in-depth pandemic risk analyses (7–9), there is a substantial probability that a pandemic with over a 100-million fatalities could be seeded from an undetected lab-acquired infection (LAI), if a single infected lab worker spreads infection as he moves about in the community. From the Klotz (2014) analysis, there is about a 1–30% probability, depending on assumptions, that, once infected, the lab worker will seed a pandemic. This large probability spread arises from varying the average number of people infected by an infected person between 1.4 and 3.0 (R0, in standard epidemiology notation), varying the details of commutes to and from work on public transportation, and whether infected acquaintances are quarantined before spreading infection. The Merler (2013) study, based on a computer-generated population grid of size and varying density of the Netherlands, supports our concern over a lab escape not being detected until it is too late: “there is a non-negligible probability (5–15%), strongly dependent on reproduction number and probability of developing clinical symptoms, that the escape event is not detected at all.”
Different methodologies were used in the Klotz (2014) and Merler (2013) risk analyses. Additional analyses are needed using other methodologies, such as the mathematical model employed for SARS (10), which hopefully will lead to some consensus on risk. The Klotz and Merler studies, however, are the first to raise these concerns and point to valid issues about the potential risks from a single LAI.
Given such a dire predicted outcome by the existing studies, the critical question is: what is the probability that a worker acquires an undetected infection in the lab in the first place? To answer this question, we reproduce here one part of the Klotz (2014) analysis: the probability of an escape through an LAI from at least one of the many labs expected to be involved in this research enterprise.
A 2013 Centers for Disease Control report is a significant source of recent data on LAIs (11). The report documents four undetected or unreported LAIs in registered US Select Agent, high-containment BSL-3 labs between 2004 and 2010. An undetected or unreported LAI implies an escape when the infected person leaves the lab. The report identifies an average of 292 registered Select Agent BSL-2, BSL-3, and BSL-4 labs operating over those 7 years, for a total of 292 × 7 = 2,044 lab years. Unfortunately, the study does not break down numbers into BSL-2, BSL-3, and BSL-4 labs or lab years.
Thus, the probability of escape for a single year, p1, can only be calculated as 4 LAIs/2,044 lab years = 0.002 or 0.2% per lab per year. This is clearly an underestimate since BSL-2 and BSL-4 labs contribute to the denominator. (The denominator used here, 2,004, equals the number of BSL-2 plus number of BSL-3 plus number of BSL-4 labs. But the denominator in our calculation should be just the number of BSL-3 labs, so the denominator is overestimated and the percent escape is then underestimated. Although requested, the CDC has not supplied us with the number of BSL-3 labs for us to do the exact calculation.) This basic probability is consistent with that for SARS escapes in Asia through LAIs (12) and with all known escapes from BSL-4 labs in the Soviet Union from LAIs and Great Britain from a mechanical failure (13).
To illustrate potential risk, the probability of no escape from a single lab in a single year is (1 − p1), so
pno=(1− p1)N × Y (1)
is the probability of no escape from N labs in Y years. And
pat least one= 1−(1− p1)N×Y (2)
is the probability of at least one escape from N labs in Y years.
Given the Science and Nature articles listed above (1, 2), it is reasonable to assume that at least 10 labs will undertake this research and that this work would continue for 10 years, so
pat least one= 1−(1−0.002)10×10= 0.18 (3)
or an 18% likelihood of at least one escape from at least one lab for the whole research enterprise, almost 100-times greater than the likelihood for a single lab in a single year.
We noted above that the probability p1 = 0.2% is conservative, estimated from the CDC data alone. The first Department of Homeland Security risk assessment for the planned National Bio- and Agro-Defense Facility in Manhattan, Kansas estimated a significantly higher escape risk, over 70% likelihood for the 50-year life of the facility (14), which works out to be a basic probability of escape, p1 = 2.4% per year. The National Research Council (14) overseeing the risk assessment remarked “The … estimates indicate that the probability of an infection resulting from a laboratory release of FMDv from the NBAF in Manhattan, Kansas approaches 70% over 50 years (see Figure 3-1) with an economic impact of $9–50 billion. The committee finds that the risks and costs could well be significantly higher than that…” While the DHS subsequently lowered the escape risk to 0.11% for the 50-year lifetime (14), the NRC committee (14) was highly critical of the new calculations: “The committee finds that the extremely low probabilities of release are based on overly optimistic and unsupported estimates of human error rates, underestimates of infectious material available for release, and inappropriate treatment of dependencies, uncertainties, and sensitivities in calculating release probabilities.” We have more trust in the NRC committee conclusions, as they have no skin in the game.
With this higher number, which we take as a worst-case scenario, the likelihood of at least one escape from 10 labs in 10 years becomes 91%, almost a certainty. It follows that, if the likelihood of one LAI leading to a pandemic is 30% in the worst-case scenario, the likelihood of an LAI-caused pandemic resulting from this whole research enterprise could be as high as 30 × 91% = 27%, a likelihood that is too dangerous to live with, as we noted. While this represents a worst-case scenario, it is not improbable.
Recent self-reported mistakes at the CDC (15), involving a particularly deadly strain of anthrax removed from BSL-3 containment and H5N1 Asian bird flu released from the CDC laboratories altogether, lend support to our concern that the probability of escape may be much greater than the 0.2% per lab per year from just LAIs. The CDC report spawned a congressional inquiry (16) and led to dozens of newspaper articles with concerns about lack of safety in high-containment laboratories.
Our concern is shared by many virologists and epidemiologists. A recent letter to the President of the European Commission (17) co-signed by 56 scientists from more than a dozen countries warned, “The probabilities of a lab accident that leads to a global spread of an escaped mutated virus are small but finite, while the impact of global spread could be catastrophic.” The European Centre for Disease Prevention and Control (18) weighed-in with its concerns as well, as did the Cambridge Working Group (19). It must be noted that some of the signers of the European Commission letter and the Cambridge Working Group’s consensus statement are the same.
The risk of a man-made pandemic from a lab escape is not hypothetical. Lab escapes of high-consequence pathogens resulting in transmission beyond lab personnel have occurred (20, 21). The historical record reveals lab-originated outbreaks and deaths due to the causative agents of the 1977 pandemic flu, smallpox escapes in Great Britain, Venezuelan equine encephalitis in 1995, SARS outbreaks after the SARS epidemic, and foot and mouth disease in the UK in 2007. Ironically, these labs were working with pathogens to prevent the very outbreaks that they ultimately caused.
Do benefits outweigh risks? Those who support PPP experiments either believe the probability of PPP escape is infinitesimal or the benefits in preventing a pandemic are great enough to justify the risk. In making decisions for what lines of research will lead to new knowledge, experts must rely on intuition honed by years of research in a particular field. In the case of this PPP research, in our opinion it would take extraordinary benefits and significant reduction of risk via extraordinary biosafety measures to correct such a massive overbalance of highly uncertain benefits to too-likely risks (Wain-Hobson, 2013).
Whatever number we are gambling with, it is clearly far too high a risk to human lives. This Asian bird flu virus research to develop strains transmissible via aerosols among mammals, and perhaps some other PPP research as well, should for the present be banned. We must emphasize that we have been considering only a very small subset of pathogen research. Most pathogen research should proceed unimpeded by unnecessary regulations.
Special precautions in BSL-4 laboratories for work with PPPs should be adopted (22). These would include:
• Training a full-time technical staff for work with PPPs. Experiments could be directed by scientists outside the laboratory using modern audio-video technology.
• Requiring the staff to follow up extended work shifts with periods of quarantine before they leave the containment area to assure that no PPP escapes from the containment area through an LAI.
• Restricting these PPP laboratories to remote locations, where an aerosol escape or other containment failure would pose the least risk of infecting an outside community.
We label BSL-4 laboratories with the special precautions, BSL-4+. While PPP experiments would be carried out primarily under BSL-4+ containment, BSL-3 containment with the special precautions might suffice for some work.
Given the global threat, the international community should insist on discussions leading to an international agreement that would require the strictest oversight to conduct this particular research anywhere. To place responsibility with the international community where it belongs and to provide maximum transparency, policy makers should require that international inspectors have access to facilities at any time on short notice.
As it stands, there is no proactive oversight nor regulations for this PPP research, so any and all of the world’s nations can carry out this dangerous work without regard to consequences. But consequences would be shared by all of us. In the meantime, insurance companies who routinely provide insurance for biological research should consider excluding such risky research from coverage.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Fouchier RA, Kawaoka Y, Cardona C, Compans RW, García-Sastre A, Govorkova EA, et al. Gain-of-function experiments on H7N9. Science (2013) 341:612–3. doi: 10.1126/science.1243325
2. Fouchier RA, Kawaoka Y, Cardona C, Compans RW, Fouchier RA, García-Sastre A, et al. Avian flu: gain-of-function experiments on H7N9. Nature (2013) 500:150–1. doi:10.1038/500150a
3. Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science (2012) 336:1534–41. doi:10.1126/science.1213362
4. Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature (2012) 486:420–8. doi:10.1038/nature10831
5. Zhang Y, Zhang Q, Kong H, Jiang Y, Gao Y, Deng G, et al. H5N1 hybrid viruses bearing 2009/H1N1 virus genes transmit in guinea pigs by respiratory droplet. Science (2013) 340:1459–63. doi:10.1126/science.1229455
6. Sutton TC, Finch C, Shao H, Angel M, Chen H, Capua I, et al. Airborne transmission of highly pathogenic H7N1 influenza in ferrets. J Virol (2014) 88:6623–35. doi:10.1128/JVI.02765-13
7. Klotz LC. The Human Fatality and Economic Burden of a Man-made Influenza Pandemic: A Risk Assessment. The Center for Arms Control & Non-Proliferation (2014). Available from: http://armscontrolcenter.org/The_Human_ ... 1-5-14.pdf or http://bio-security.org/wp-content/uplo ... 1-5-14.pdf
8. Klotz L, Sylvester S. Opinion: The Fatality Burden. (2013). Available from: http://www.the-scientist.com/?articles. ... ty-Burden/
9. Merler S, Ajelli M, Fumanelli L, Vespignani A. Containing the accidental lab escape of potential pandemic influenza viruses. BMC Med (2013) 11:252. doi:10.1186/1741-7015-11-252
10. Lipsitch M, Cohen T, Cooper B, Robins JM, Ma S, James L, et al. Transmission dynamics and control of severe acute respiratory syndrome. Science (2003) 300:1966–70. doi:10.1126/science.1086616
11. Henkel RD, Miller T, Weyant RS. Monitoring select agent theft, loss and release reports in the United States 2004-2010. Appl Biosafety (2012) 17:171–80. Available from: http://www.absa.org/abj/abj/121704FAHenkel.pdf
12. Specific Incidences of Lab-Acquired SARS-CoV Infections. Centers for Disease Control and Prevention (2005). Available from: http://www.cdc.gov/sars/lab/biosafety.html
13. Klotz LC. Sharpening Our Intuition on Man-made Pandemics. Breeding BioSecurity Blog (2012). Available from: http://bio-security.org/wp-content/uplo ... on0515.pdf
14. Evaluation of the Updated Site-Specific Risk Assessment for the National Bio- and Agro-Defense Facility in Manhattan, Kansas. The National Academies Press (2012). Available from: http://www.nap.edu/catalog.php?record_id=13418
15. Report on the Potential Exposure to Anthrax. Centers for Disease Control and Prevention (2014). Available from: http://www.cdc.gov/od/science/integrity ... Report.pdf
16. Review of CDC Anthrax Lab Incident. Energy & Commerce Committee, United States House of Representatives. (2014). Available from: http://energycommerce.house.gov/hearing ... dent#video
17. Wain-Hobson S. Response to Letter by the European Society for Virology on “Gain-of-Function” Influenza Research. The Foundation for Vaccine Research (2013). Available from: http://www.nature.com/polopoly_fs/7.145 ... letter.pdf
KNAW has invited two scientists who contributed to the debate on gain-of-function by writing a letter to the president of the EU-Commission, Mr Barroso. In his capacity as chairman of the European Society for Virology, professor Giorgio Palù, asked attention for the potential benefits of this kind of research. Professor Simon Wain-Hobson, chairman of the Foundation for Vaccine Research, responded with a letter undersigned by 56 other scientists -- that challenged the arguments in favour. In their opening statements both Dr Palù and Dr Wain Hobson will explain and elaborate their main arguments.
Following their presentations both speakers will –- for the first time -– have a debate with each other. Of course there will be attention for the more scientific aspects of gain-of-function research. However, the political and societal debate was not only –- and probably not even in the first place – on science, but even more on the security aspects. One of the reasons for the debate is the risk of bioterrorism: the creation of a pandemic by terrorists having the knowledge and the biological agents. Since the attack with anthrax letters in the USA life scientists know -– or should know -– that this is a real issue of concern. How to deal with it, as science and as society?...
PRESENTATION SIMON WAIN-HOBSON
His main thesis for this debate is as follows: gain-of-function avian influenza research is frighteningly out of touch. The flu that worries us is that among humans. Influenza viruses are characterized by two kinds of proteins called H (hemagglutinin) and N (neuraminidase) at the surface of the virus that allow it to get into and out of cells. Human pandemics have been provoked by H1N1 (Spanish flu 1918, 1977 and 2009), H2N2 (Asian flu, 1957) and H3N2 viruses (Hong Kong flu, 1968). By contrast the greatest reservoir of influenza viruses is among ducks, birds and chickens and they rarely cross over to humans. Occasionally an epidemic of avian flu spills over to humans and can give rise to hundreds of H7N9 infections in man in a matter of months. Fortunately, these viruses are almost never transmitted from human to human. This is the same as with the rabies virus. Such dead end infections are not new in virology. The question is could these viruses become transmittable between humans and if so how? In modern virological parlance, what mutations are necessary to convert such a virus into a highly transmissible virus between ferrets, the animal of choice in influenza biology. This is the basis of the H5N1 projects of Fouchier and Kawaoka. As is now well known, they succeeded in doing this for the H5N1 virus and it involved a handful of mutations.
It should be emphasized that the conditions in a laboratory differ enormously from those in nature. Influenza virus evolution takes years, while in a laboratory there is a massive acceleration thanks to the selection of mutants by the virologist. To illustrate this, suffice to say that for some reason nature has not yet succeeded in morphing any influenza virus into a major human pathogen other than H1N1, H2N2 and H3N2 in 100 years. Even the resurrection of the Spanish flu HIN1 virus did not help us to predict or prepare for the H1N1 flu pandemic of 2009.
This is one of the major scientific weaknesses in the so-called gain-of-function influenza research. We do not know if the experiments reflect what happens in nature. As an HIV expert he can say this because the lab is not a good template for what happens in the clinic. There are hundreds of possible trajectories and only a few can be tested in laboratory. One can never know which are the “right ones” – that can only be appreciated as being “right” once the pandemic has struck. As for obvious ethical reasons these experiments cannot be done in humans, an important objection to this work is that it is not falsifiable. This constitutes a real issue as science tries to solve problems, not to create them.
He gave two examples of quantum jumps in recent gain-of-function influenza research. An ostrich H7N1 virus was selected to become droplet transmissible between ferrets. It did not lose lethality -- three out of five animals died (Sutton et al., J Virology 88, 6623 (2014)). A 60% lethality rate is much greater than the 2% that characterized Spanish flu. We must realize that until now no case of H7N1 in humans has been reported, so it is no threat to humans. The world is becoming a more dangerous place by making the virus aerosol transmittable. The second example is an unpublished experiment from Dr Kawaoka. He engineered the human 2009 pandemic H1N1 virus to escape neutralization by convalescent sera. By doing this the virus escaped vaccine coverage. It needs no explanation that if there ever were a lab accident the consequences could be enormous because this virus is unquestionably transmissible between humans.
The benefits of this kind of research: Scientists are used to work with the probability of benefits versus risks, just as they are used to handling dangerous agents. One of the arguments in favour of this gain-of-function research is developing vaccines and drugs. GOF research has nothing to offer for making vaccines. Development of vaccines is a very time-consuming and expensive effort. Moreover, each strain of influenza virus would need its own vaccine. And as mentioned above there is a great number of possible strains. And as it cannot be predicted which strain will cause an epidemic, this kind of research does not have much utility. Developing and stockpiling these vaccines would cost an enormous amount of money. As for drugs the choice is binary: we must hope that the next pandemic strain is sensitive to available drugs and use them accordingly. If the strain is resistant, it will take too long for the new drugs to be tested -– by then the pandemic will be over.
Now the risks of gain-of-function research: There is always the risk of an accident. For a virus that the human system has never seen this could lead to millions of deaths. Wain-Hobson does not call this risk, but catastrophic risk. But there are also less catastrophic outcomes, e.g. the equivalent of a seasonal flu outbreak where mortality is less than a million. The economic burden of seasonal flu costs a lot of money (say $71-167 bn/year to US economy alone). What would be special in the event of release of a lab engineered virus is that it is genetically ‘barcoded’. It would be trivial to trace the origin. Massive liability claims would be sought for the unwarranted deaths and economic damage. Liability experts (e.g. from RAND corporation) say that this really could lead to claims of 100 billion dollars and more, an amount that no insurance company would be willing, or able, to pay. Liability of this magnitude would jeopardize the existence of private universities and institutions such as the Institut Pasteur or even Harvard.
Some scientists argue that there is not much guidance on these biosafety issues. Of course there is the Fink report of 2004 (NRC -- National Research Council (2004), Biotechnology Research in an Age of Terrorism. Washington DC: National Academies of Science). And there is the InterAcademy Panel 2007 Statement on Biosecurity. This statement has been signed by 76 Academies of Sciences, such as KNAW, Royal Society, Leopoldina and the US NAS. Basically this statement says that scientists have an obligation to do no harm (the Hippocratic oath). It also says that individual good conscience of scientists does not imply that one can ignore the possibility of misuse. However, many scientists do so. Scientists are responsible for the knowledge they produce and publish. The statement also refers to the necessity of oversight over the research. This certainly is relevant for funders but also journal editors should adhere to these principles in making their decisions on the publication of dual use research. Amazingly hardly anyone knows about this document. It is good to have such a statement, but it is important that people know about it! So, maybe there is not so much a lack of guidance, but a lack of good communication of that guidance. For the moment scientists effectively transfer their biosafety and biosecurity problems to journal editors who have to handle them at “5 minutes before midnight”.
From his point of view we should freeze on gain-of-function research, because not doing so means waiting for an accident. With this breathing space virologists can look for more coordination and cooperation in taking safety and security measures, because they are very divergent now. Very important –- and Giorgio Palù and Simon Wain-Hobson agree on this -– is having more debates with more stakeholders getting in; lawyers, defence experts, insurance companies, etc. We need more risk–benefit analysis, although with the disagreement among virologists, it seems hard to get an agreement on the benefits. For the moment learned societies, governments and funders do little to advance this field. They fund and support this research without taking care of risk or liability.
Wain-Hobson finishes with this statement: On 22 March 2012, Russian president-elect Vladimir Poutin [Putin] promised to develop new weapons based on advanced technologies, including genetics (Raymond Zilinskas, The Soviet biological warfare program and its uncertain legacy, Microbe v9, p151, 2014). Although the Soviet Union was one of the initiators and first signatories and depository state of the Biological and Toxin Weapons Convention, there are clear signals that they went on with research and development of biological weapons until 1990. It has never been verified, because that was not possible for political reasons. So, how do we understand this recent statement from Poutin [Putin]? Bluff or a serious warning?
Virology is hopelessly misguided in pursuing gain-of-function research. It will not lead help us predict or prevent influenza. Although virology has made great advances, it is a dream that is still years away. Indeed flu viruses may always be one step ahead of us precisely because they evolve to escape immunity to them. As long as that is the case debate is needed, among virologists, but also with experts from other fields.
-- Gain-Of-Function Research: Report of a Debate between Prof. Giorgio Palù and Prof. Simon Wain-Hobson, 25 June 2014, Trippenhuis, Amsterdam, by Koos van der Bruggen
18. Circulating Avian Influenza Viruses Closely Related to the 1918 Virus Have Pandemic Potential. European Centre for Disease Prevention and Control. (2014). Available from: http://ecdc.europa.eu/en/activities/sci ... d72&ID=765
Circulating Avian Influenza viruses closely related to the 1918 virus Have pandemic potential
by European Centre for Disease Prevention and Control
July 16, 2014
A recent article by Watanabe et al. in the Cell Host & Microbe journal describes an attempt to assess the risk of emergence of pandemic influenza viruses closely related to the 1918 influenza virus.
Reverse genetics methods were used to generate an avian influenza virus closely related to the 1918 influenza virus, based on sequence information reported from various avian influenza viruses. Further experiments were done to demonstrate what mutations are required for this virus to become easily transmissible between mammals. Effectiveness of the current influenza vaccine and the antiviral drug oseltamivir against the 1918-like influenza virus was also assessed.
It was experimentally demonstrated that a 1918-like avian influenza virus with a limited number of additional mutations exhibits relatively high pathogenicity in mammals, although lower than the original 1918 influenza virus strain that was also recreated with reverse genetics technology in the laboratory. In the transmissibility studies, only some constellations of the virus genes allowed transmissibility between ferrets, suggesting roles for RNA replication complex, HA and NA in virus transmission.
To further assess the risk of the emergence of such avian influenza viruses that could infect humans, the team examined the prevalence of avian influenza viruses that are similar to the 1918 influenza virus, and looked for avian influenza viruses possessing human-type amino acid residues.
The study thus demonstrated the continued circulation of such avian influenza viruses that possess 1918 virus-like proteins and viruses that may acquire 1918 virus-like properties. This would suggest that a potential exists for a 1918-like pandemic virus to emerge from the avian virus gene pool.
ECDC comment
The study by Watanabe et al. is the latest in a series of genetic engineering experiments where research groups have created influenza viruses of pandemic potential. The new study discussed here further confirms the power of recombinant technology to create pathogenic viruses that are not currently circulating in nature. From the public health perspective, this poses a risk both for the laboratory personnel working with these viruses, even in very secure biosafety conditions, and to the general public in case of a laboratory escape. Recent incidents remind us that laboratory accidents and laboratory escapes can happen with dangerous pathogens, even if the highest security standards are applied [1,2,3]. The developments in technology should not and cannot be stopped and research laboratories should have the freedom to apply the latest technologies for science purposes as long as this is done with full adherence to good ethical and biosafety practices. However, the decision to fund this type of gain of pathogenic function studies in influenza viruses and other human pathogens has stirred scientific controversy about the mechanisms currently in place to ensure sound assessment of risk and benefits by independent reviewers [4].
The public health perspective to the critical review of funding such dual-use research of concern including studies aiming at the creation of potential pandemic pathogens has been so far limited. Very often the research groups justify their research agenda with pandemic preparedness and better understanding of the avian influenza viruses without further specifying how exactly the results may improve the preparedness plans. It is important to ask what this type of result adds to the field of pandemic influenza preparedness and how the prediction of efficacy of influenza vaccines or antivirals against influenza viruses is improved based on these results. Furthermore, it is pertinent to ask for justification of the methods, and if any of those could be replaced by safer experiments. It is of utmost importance that the biosafety practices and controls in the laboratories undertaking such research are kept to a high standard. A forum for public health discussion around dual-use research of concern topics is not yet available at European level. ECDC advocates for open discussion about studies where potential pandemic threats are created. The research community should in all their work apply the medical ethical principle of “first do no harm”.
19. Cambridge Working Group Consensus Statement on the Creation of Potential Pandemic Pathogens (PPPs). Available from: http://www.cambridgeworkinggroup.org/
Cambridge Working Group Consensus Statement on the Creation of Potential Pandemic Pathogens (PPPs)
by cambridgeworkinggroup.org
July 14, 2014
Recent incidents involving smallpox, anthrax and bird flu in some of the top US laboratories remind us of the fallibility of even the most secure laboratories, reinforcing the urgent need for a thorough reassessment of biosafety. Such incidents have been accelerating and have been occurring on average over twice a week with regulated pathogens in academic and government labs across the country. An accidental infection with any pathogen is concerning. But accident risks with newly created “potential pandemic pathogens” raise grave new concerns. Laboratory creation of highly transmissible, novel strains of dangerous viruses, especially but not limited to influenza, poses substantially increased risks. An accidental infection in such a setting could trigger outbreaks that would be difficult or impossible to control. Historically, new strains of influenza, once they establish transmission in the human population, have infected a quarter or more of the world’s population within two years.
For any experiment, the expected net benefits should outweigh the risks. Experiments involving the creation of potential pandemic pathogens should be curtailed until there has been a quantitative, objective and credible assessment of the risks, potential benefits, and opportunities for risk mitigation, as well as comparison against safer experimental approaches. A modern version of the Asilomar process, which engaged scientists in proposing rules to manage research on recombinant DNA, could be a starting point to identify the best approaches to achieve the global public health goals of defeating pandemic disease and assuring the highest level of safety. Whenever possible, safer approaches should be pursued in preference to any approach that risks an accidental pandemic.
Original Signatories & Founding Members:
(Founding Members met in Cambridge on July 14 and crafted the statement)
Amir Attaran, University of Ottawa
Barry Bloom, Harvard School of Public Health
Arturo Casadevall, Albert Einstein College of Medicine
Richard Ebright, Rutgers University
Nicholas G. Evans, University of Pennsylvania
David Fisman, University of Toronto Dalla Lana School of Public Health
Alison Galvani, Yale School of Public Health
Peter Hale, Foundation for Vaccine Research
Edward Hammond, Third World Network
Michael Imperiale, University of Michigan
Thomas Inglesby, UPMC Center for Health Security
Marc Lipsitch, Harvard School of Public Health
Michael Osterholm, University of Minnesota/CIDRAP
David Relman, Stanford University
Richard Roberts (Nobel Laureate '93), New England Biolabs
Marcel Salathé, Pennsylvania State University
Lone Simonsen, George Washington University
Silja Vöneky, University of Freiburg Institute of Public Law, Deutscher Ethikrat
Charter Members:
(Charter Members of the Cambridge Working Group endorse the statement)
Porter W. Anderson Jr (Lasker laureate '96), Harvard Medical School & University of Rochester
Roy Anderson, Imperial College London, UK
Rustom Antia, Emory University
Ann Arvin, Stanford University School of Medicine
Birgitta Åsjö, University of Bergen, Norway
John Bartlett, Johns Hopkins University School of Medicine
Patrick Berche, Necker-Enfants Malades Medical School, Paris, France
Paul Berg (Nobel laureate '80), Stanford University
Pamela Björkman, California Institute of Technology, Pasadena
Steven Black, Center for Global Health, Cincinnati Children's Hospital
Jesse D. Bloom, Fred Hutchinson Cancer Research Center
Sebastian Bonhoeffer, ETH Zürich, Switzerland
Michel Brahic, Department of Genetics, Stanford University School of Medicine
Christian Bréchot, Institut Pasteur, Paris
Sydney Brenner (Nobel laureate '02)
Roger Brent, Fred Hutchinson Cancer Research Center
John S. Brownstein, Harvard Medical School
Donald S. Burke, University of Pittsburgh
Dennis Burton , Scripps Research Institute, La Jolla
Donald Coen, Harvard Medical School
Vittorio Colizzi, University of Rome Tor Vergata, Italy
Larry Corey, Fred Hutchinson Cancer Research Center
Roel Coutinho, University Medical Center Utrecht, The Netherlands
Pascale Cossart, Institut Pasteur, France
Clyde S. Crumpacker, Beth Israel Deaconess Medical Center
Malcolm Dando, University of Bradford, UK
Norman Daniels, Harvard School of Public Health
Patrice Debré, Hôpital Pitié Salpetrière, Paris
Jonathan A. Eisen, University of California, Davis
Santiago Elena, CSIC, Spain
Max Essex (Lasker laureate '86), Harvard School of Public Health
Stanley Falkow (Lasker laureate '08), Stanford University School of Medicine
Michael Farzan, Scripps Research Institute, Miami
Marcus W. Feldman, Stanford University
Neil M. Ferguson, Imperial College, UK
Adam Finn, University of Bristol, UK
Christophe Fraser, Imperial College, UK
Julio Frenk, Harvard School of Public Health
José Gatell, Hospital Clínic, University of Barcelona, Spain
Gabriela Gomes, Instituto Gulbenkian de Ciencia, Portugal
Eric P. Goosby, University of California San Francisco
Nathalie Grandvaux, Université de Montréal, Canada
Warner C. Greene, Gladstone Institute of Virology and Immunology, University of California San Francisco
Jeanne Guillemin, MIT
Willem Hanekom, Bill & Melinda Gates Foundation, and University of Cape Town, South Africa
Elisa D. Harris, Center for International and Security Studies at Maryland
Eric Harvill, Pennsylvania State University
Daniel L. Hartl, Harvard University
Donald A. Henderson (Presidential Medal of Freedom '02), University of Pittsburgh
Martin S. Hirsch, Harvard Medical School
Richard Jackson, University of Cambridge
Laura H. Kahn, Princeton University
Phyllis Kanki, Harvard School of Public Health
Lynn Klotz, Center for Arms Control & Non-Proliferation
Keith Klugman, Bill & Melinda Gates Foundation
Roberto Kolter, Harvard Medical School
Mathilde Krim, Founding Chairman, amfAR, The Foundation for AIDS Research
Leonid Kruglyak, UCLA
Richard E. Lenski, Michigan State University
Matti Lehtinen, University of Tampere, Finland
Bruce R. Levin, Emory University
Gunnar Lindahl, Lund University, Sweden
W. Ian Lipkin, Columbia University
Ian M. Mackay, University of Queensland, Australia
Adel A. Mahmoud, Princeton University
Rick Malley, Boston Children's Hospital
Howard Markel, University of Michigan
Robert M. May (former president Royal Society), University of Oxford, UK
Mike McCune, University of California, San Francisco
Kenneth McIntosh, Boston Children’s Hospital
Andrew McMichael, University of Oxford
Andreas Meyerhans, ICREA & Universitat Pompeu Fabra, Barcelona, Spain
Julio S. G. Montaner, University of British Columbia
Jonathan D. Moreno, University of Pennsylvania
Richard Moxon, Oxford University, UK
Neal Nathanson, Microbiology, University of Pennsylvania
Kathryn Nixdorff, Darmstadt University of Technology, Germany
Kate O'Brien, Johns Hopkins Bloomberg School of Public Health
Paul Offit, The Children's Hospital of Philadelphia
Gérard Orth, Institut Pasteur, France
Eli Perencevich, University of Iowa
Joshua B. Plotkin, University of Pennsylvania
Stanley Plotkin, University of Pennsylvania
Peter Piot, London School of Hygiene & Tropical Medicine, UK
Mark Poznansky, Harvard Medical School, Massachusetts General Hospital
Andrew Rambaut, University of Edinburgh, UK
Johannes Rath, University of Vienna, Austria
Andrew Read, Pennsylvania State University
Gili Regev-Yochay, Sheba Medical Center, Israel
Félix Rey, Institut Pasteur, Paris, France
Douglas D. Richman, University of California San Diego
Steven L. Salzberg, Johns Hopkins School of Medicine
Philippe Sansonetti, Institut Pasteur, Paris
Mathuram Santosham, Johns Hopkins University
Mauro Schechter, Projeto Praca Onze, Universidade Federal do Rio de Janeiro, Brazil
Olivier Schwartz, Institut Pasteur, Paris, France
Jeffrey Shaman, Columbia University
Kai Simons, Max Planck Institute of Molecular Cell Biology & Genetics, Dresden, Germany
Stephen C. Stearns, Yale University
Tomoko Steen, Georgetown University Medical School
John Steinbruner, University of Maryland
Bruce Stillman, Cold Spring Harbor Laboratory
Klaus Stöhr, Novartis Vaccines and Diagnostics
Amalio Telenti, University of Lausanne, Switzerland
Paul Turner, Yale University
Robert D. Truog, Harvard Medical School
Ross Upshur, University of Toronto, Canada
Paul Volberding, AIDS Research Institute, University of California San Francisco
Mark Wainberg, McGill University, Montreal, Canada
Simon Wain-Hobson, Institut Pasteur, France
James D. Watson (Nobel laureate '62)
David Weiner, University of Pennsylvania
Robin Weiss, University College London, UK
Hans Wigzell, Karolinska Institutet, Stockholm, Sweden
Daniel Wikler, Harvard University
Claus O. Wilke, The University of Texas at Austin
Rüdiger Wolfrum, Max Planck Institute, Germany
Priscilla Yang, Harvard Medical School
Moshe Yaniv, Institut Pasteur, France
Patrick Yeni, Hôpital Bichat and University of Paris 7 Paris, France
Jerome A. Zack, David Geffen School of Medicine, University of California Los Angeles
Charter Members who signed the statement online:
Andrew Noymer, University of California, Irvine
Maimuna S. Majumder, MIT
Christopher McCabe, University of Alberta, Canada
Mike McCormick, Labwatch
Nicole E Basta, Princeton University
Steven Riley, Imperial College London
Ben Ashby, University of Exeter
Gautam I Menon, The Institute of Mathematical Sciences, Chennai, INDIA
Ted Cohen, Harvard School of Public Health
Toby Ord, Oxford University
Sean O hEigeartaigh, University of Oxford
Daniel Dewey, University of Oxford
Joann Mead, @JoannJMead
Christian Althaus, Institute of Social and Preventive Medicine (ISPM), University of Bern, Switzerland
Marius Gilbert, Université Libre de Bruxelles
Joshua S. Weitz, Georgia Institute of Technology
Charles N Haas, Drexel University
R. Taylor Raborn, Indiana University
Amy Greer, University of Guelph
Brett Edwards, University of Bath
Antoni Trilla, Hospital Clinic - Univ. of Barcelona
David S. Fedson, Sergy Haut, France
Hortense Gerardo, Lasell College
Joseph Baglieri, Barrister & Solicitor
Dawn Kubly, Fort Healthcare
Charles R. Stack, University of Illinois at Chicago School of Public Health
Lisa Murillo, Los Alamos National Laboratory
Anders Sandberg, Oxford University
Evan Skowronski, TMG Biosciences
Kathleen Gilbert, California Institute of Technology
Mukilan D Suresh, SRM University, School of Bioengineering (student)
Roberto Anitori, Clark College, WA, USA
Andrew Leifer, Princeton University
Pankaj Mehta, Boston University
Megan Murray, Harvard Medical School
Eric S. Starbuck, Department of Health & Nutrition, Save the Children USA
Carl T. Bergstrom, Department of Biology, University of Washington
Nicola Low, University of Bern, Switzerland
Sünje J. Pamp, Technical University of Denmark
Andrew Snyder-Beattie, University of Oxford
Viktor Müller, Eötvös Loránd University and the Hungarian Academy of Sciences, Budapest
Tami Lieberman, Harvard Medical School
Alan Barbour, University of California Irvine
Sven Bergstrom, Umea University, Sweden
Sophia Roosth, Assistant Professor of the History of Science, Harvard University
Sibel Ascioglu, Hacettpe University, Dept. of Infectious Diseases, Turkey
Janet D. Stemwedel, San José State University
Fernando Baquero, Department of Microbiology, Ramón y Cajal Institute for Health Research (IRYCIS) Madrid, Spain
Carlos S. Moreno, Emory University
Sarah Otto, University of British Columbia
Richard Levins, Harvard School of Public Health
Andrew Tatem, University of Southampton
Benjamin Kerr, University of Washington
A. David Paltiel, Yale School of Public Health / Yale School of Management
Rochelle Walensky, Massachusetts General Hospital
John W. Jackson, Brigham and Women's Hospital
Julie Parsonnet, Infectious Diseases, Stanford University School of Medicine
John McGowan, Emory University Rollins School of Public Health
John Quackenbush, Dana-Farber Cancer Institute and Harvard School of Public Health
Rafael Canton, Servicio de Microbiologia. Hospital Universitario Ramón y Cajal. Madrid. Spain
Jane Kim, Harvard School of Public Health
Thomas F O'Brien, Brigham and Women's Hospital, Boston, MA
Simon Levin, Princeton University
James Lloyd-Smith, UCLA
Andrew Yates, University of Glasgow
Richard C. Larson, Massachusetts Institute of Technology
Victor DeGruttola, Harvard University
Melinda M. Pettigrew, Yale School of Public Health
Marc D. Natter, Harvard Medical School
Ramanan Laxminarayan, Center for Disease Dynamics, Economics & Policy, New Delhi
Cynthia Schuck-Paim, Origem Scientifica
Emma McBryde, University of Melbourne & Victorian Infectious Diseases Service, Doherty Institute
Robin Bush, University of California, Irvine
Pieter Trapman, Stockholm University
Lumi Viljakainen, University of Oulu
Isabelle Magnoli, Université catholique de Louvain
Jeremy Van Cleve, Duke University
Francisco Dionisio, University of Lisbon
Daniel Wilson, University of Oxford
Amy Hurford, Memorial University of Newfoundland
Sergios-Orestis Kolokotronis, Fordham University
Pedro Silva, University of Lisbon, Portugal
Oscar E. Gaggiotti, University of St Andrews, UK
Richard Edwards, University of New South Wales
Emilia Vynnycky, London School of Hygiene & Tropical Medicine, UK
Martin Kulldorff, Harvard Medical School
Erik F. Y. Hom, University of Mississippi
John Mekalanos, Harvard Medical School
Stephen W. Schaeffer, Pennsylvania State University
Christina Davy, Trent University, Peterborough, Ontario, Canada
Clark Freifeld, Boston Children's Hospital
David A Harmin, Harvard Medical School
Anne D. Yoder, Duke University
Nicholas G Reich, University of Massachusetts - Amherst
Gerald Learn, University of Pennsylvania
Pleuni Pennings, San Francisco State University
Zachary A Szpiech, University of California, San Francisco
Stan Finkelstein, MIT/Harvard Medical School
Paul Avillach, Harvard Medical School
Alain-jacques Valleron, Inserm. Paris, France
Ellen L. Simms, University of California, Berkeley
Anahi Espindola, University of Idaho
Claudio Jose Struchiner, Oswaldo Cruz Foundation, Brazil
Jan Medlock, Oregon State University
Maarten Vonhof, Western Michigan University
Heather Douglas, University of Waterloo
Michael Höhle, Department of Mathematics, Stockholm University
Joy Scaria, Cornell University
Preben Aavitsland, Epidemi, Kristiansand
Fernando Gonzalez-Candelas, University of Valencia, Spain
Dr. Sameeh Ghazal, King Fahad Medical City
William J. Etges, University of Arkansas
Sadia Shakoor, Aga Khan University
Angela Hancock, Max F. Perutz Labs, University of Vienna
Michael Merson, Duke University
Donald R. Olson, New York City Department of Health and Mental Hygiene
Robert Gordon White, Carleton University
Paul Schmid-Hempel, ETH Zurich
Matt Keeling, University of Warwick
Victoria Shaffer, Infection Preventionist; CHWC, Bryan, Ohio
Matthew J. Brown, University of Texas at Dallas
Joel L. Sachs, University of California - Riverside
Jacques Dubochet, University of Lausanne (retired)
François-Xavier Weill, Institut Pasteur, Paris, France
Rodrigo Angerami, State University of Campinas/UNICAMP
Omar Cornejo, Washington State University
Wladimir J. Alonso, Origem Scientific Consultancy
Jacob-S. Seeler, Institut Pasteur, Paris
Sarah Cobey, University of Chicago
Joshua Metlay, Massachusetts General Hospital/Harvard Medical School
Barbara E. Giles, Umea University, Sweden
Brigitte Autran, University Pierre et Marie Curie, Paris
Nicholas S Kelley, University of Minnesota
Jean-Michel Guillon, University of Paris Sud, France
Jon Zelner, Princeton University, Dept. of Ecology and Evolutionary Biology
William C. Miller, UNC - Chapel Hill
Katia Koelle, Duke University
Thomas W. Jeffries, University of Wisconsin - Madison
Harry Greenberg, Stanford University
Romain Gallet, INRA - Montpellier (France)
Allan Hance, INSERM, Paris, France
Simon Fellous, INRA - CBGP, France
Tatyana Novossiolova, University of Bradford
Patricia Baldacci, Institut Pasteur
Godwin Wilson, Hamad Medical Corporation, Qatar
Ralf Koebnik, Institut de Recherche pour le Développement
Elaine Nsoesie, Boston Children's Hospital, Harvard Medical School
Nicolas Voirin, Hospices Civils de Lyon
Mariet Feltkamp, Leiden University Medical Center
Conor Browne, Queen's University Belfast
Quentin Sattentau, University of Oxford
Michael Costa, Abt Associates
Jonathan A. Cooper, Fred Hutchinson Cancer Resarch Center
Andrew Lakoff, University of Southern California
Peter V. Markov, Institut Pasteur
Doug Cyr
bracha rager, faculty of health sciences, ben gurion univ. Israel
Mark Eisler, University of Bristol
Miles Davenport, UNSW Australia
David Bofinger, Grand Canyon Univerity - Graduate Student -Psychology
Daniel Leifer, UC Davis School of Medicine
Benjamin de Bivort, Harvard University
James Lyons-Weiler, PhD, Ebola Rapid Assay Development Consortium
Camillo Di Cicco, University of Rome
Roberto Bruzzone, HKU-Pasteur Research Pole
Anders Huitfeldt, Harvard T.H. Chan School of Public Health
Viktoriya Krakovna, Harvard University PhD student, Future of Life Institute cofounder
sally, uLVxnqHkrg
Emma Kowal, Harvard University
Ales Flidr, Harvard College
Max Kesin, Professional
Dr. Roman V. Yampolskiy, University of Louisville
Robert J. Taylor, Sage Analytica, Bethesda Maryland
Niel Bowerman, Future of Humanity Institute, University of Oxford
Nancy Connell, Rutgers New Jersey Medical School
Kathleen Burns, Sciencecorps
Louis Kang, Harvard University/MIT
Thomas Choate, Air
Aaron Supple, Beth Israel Deaconess Medical Center
Dominic Hall, Cambridge University, Stem Cell Institute
Niran Adeyanju, Obafemi Awolowo University, Nigeria.
Joseph (Jo) Walker, US CDC (signing in personal capacity)
Jeremy Ferranti, Researcher
Bonnie Page
NICOULAUD GHISLAINE
Alexandra Richter, DPFA-Regenbogen-Schulen
David Hartsuch, MD MS CPA, Emergency Resource
Jimmy Joseph Moise, Le P'ti Club inc.
20. Furmanski M. Lab Escapes and “Self-fulfilling prophecy” Epidemics. Center for Arms Control and Nonproliferation (2014). Available from: http://armscontrolcenter.org/Escaped_Vi ... -17-14.pdf
21. Furmanski M. Threatened pandemics and lab escapes: self-fulfilling prophecies. Bull Atom Sci (2014). Available from: http://thebulletin.org/threatened-pande ... hecies7016
22. Klotz LC, Sylvester EJ. The unacceptable risks of a man-made pandemic. Bull Atom Sci (2012). Available from: http://thebulletin.org/unacceptable-ris ... e-pandemic
- admin
- Site Admin
- Posts: 40066
- Joined: Thu Aug 01, 2013 5:21 am
Re: U.S. government gave $3.7 million grant to Wuhan lab at
Letter to The NSABB Board [U.S. National Science Advisory Board for Biosecurity]
by Lynn C. Klotz, Ph.D., Senior Science Fellow and member of the Scientist Working Group on Biological and Chemical Weapons Center for Arms Control and Non-proliferation, Washington, DC, USA
September 2015
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT
To: The NSABB Board (in advance of the September 28, 2015 meeting)
From: Lynn C. Klotz, PhD, Senior Science Fellow and member of the Scientist Working Group on Biological and Chemical Weapons Center for Arms Control and Non-proliferation, Washington, DC, USA
Home contact Information: 5 Duley Street, Gloucester MA 01930, 978-281-6015, [email protected]
The following document is a first draft of a literature-research project that started in the summer of 2015. The project is in an early stage, but has gone far enough to make its point: There is an urgent need for international proactive oversight of influenza research that might increase the pathogenicity of influenza viruses. Some of this gain-of-function research may create lab-made potential pandemic influenza viruses.
Even if the probability is small for an escape from a lab in a single year for such a virus, the fact that there are a large number of research projects underway throughout the world, projects that will be conducted for many years, the overall probability of escape from at least one lab is uncomfortably high.
The Potential Pandemic Influenza Research Enterprise
In a recent Letter to the Editor titled Danger of Potential-Pandemic-Pathogen Research Enterprises (http://intl-mbio.asm.org/content/6/3/e00815-15.full), I argued that there are likely many labs throughout the world, many not funded by the NIH, that are developing mammal-contagious influenza viruses. Research that makes avian, mammalian, or human influenza viruses more virulent, increases their transmissibility, alters their host range, or evades countermeasures is potentially dangerous and may create potential pandemic pathogens.
Influenza viruses are more likely to fuel an uncontrollable outbreak because of their long history of doing just that. This kind of research got considerable attention in 2011 when Professor Ron Fouchier announced that his laboratory had made the H5N1 highly pathogenic avian influenza virus (HPAI) airborne transmissible by respiratory aerosols from ferret to ferret.
In the context of this analysis of recent publications reported in Pub Med (references (1) through (35)), the larger category of Experiments of Concern (EoC) is used as a guide to look for potentially dangerous research. In 2004, the National Academy of Sciences published a report Biotechnology Research in an Age of Terrorism (http://www.nap.edu/catalog/10827.html). The so-called Fink Committee that produced the report was asked to “consider ways to minimize threats from biological warfare and bioterrorism without hindering the progress of biotechnology, which is essential for the health of the nation.” The committee recommended that the “Department of Health and Human Services…create a review system for seven classes of experiments (the Experiments of Concern) involving microbial agents that raise concerns about their potential for misuse.” Specifically, the EoC are:
“1. Would demonstrate how to render a vaccine ineffective. This would apply to both human and animal vaccines…
2. Would confer resistance to therapeutically useful antibiotics or antiviral agents. This would apply to therapeutic agents that are used to control disease agents in humans, animals or crops…
3. Would enhance the virulence of a pathogen or render a non-pathogen virulent. This would apply to plant, animal, and human pathogens…
4. Would increase transmissibility of a pathogen. This would include enhancing transmission within or between species. Altering vector competence to enhance disease transmission would also fall into this class.
5. Would alter the host range of a pathogen. This would include making non zoonotics into zoonotic agents. Altering the tropism of viruses would fit into this class.
6. Would enable the evasion of diagnostic/detection modalities. This could include microencapsulation to avoid antibody-based detection and/or the alteration of gene sequences to avoid detection by established molecular methods.
7. Would enable the weaponization of a biological agent or toxin. This would include the environmental stabilization of pathogens.”
These seven classes of experiments “will require review and discussion by informed members of the scientific and medical community before they are undertaken [proactive oversight] or, if carried out, before they are published in full detail.” For experiments making deadly avian influenza viruses airborne transmissible, many scientists think they should not be carried out at all.
An excellent system for reviewing potentially dangerous experiments, Controlling Dangerous Pathogens: A Prototype Protective Oversight System, was developed in 2007 by The Center for International and Security Studies at Maryland (http://drum.lib.umd.edu/bitstream/1903/ ... ograph.pdf). It recommends a tiered review, from most to least dangerous research. Paraphrased from the Maryland paper:
In my opinion, there should be two levels of local oversight. The first level is the currently employed Institutional Biosafety Committee (IBC), and the second is an outside committee. There is concern that IBCs will simply rubber-stamp research proposals from labs in their own institution, so I suggest proactive oversight by a committee outside the institution (perhaps at the state level in the US) for experiments of concern on influenza viruses that do not carry an immediate threat of an outbreak from an escape from the laboratory (e.g., vaccine viruses and other attenuated and inactivated viruses).
Because of the potential for some strains of lab-made influenza viruses to cause international outbreaks, research mutagenizing these viruses that could result in increased pathogenicity (gain of function) should be subject to external oversight. At present, there is little national and no international proactive oversight with any authority to guide or ban experiments. See for instance: Gronvall GK, Rozo M. Synopsis of Biological Safety and Security Arrangements. UPMC Center for Health Security.July 2015. Available at http://www.upmchealthsecurity.org/ourwo ... rangements.
The literature analysis
To date, only one general Pub Med search term, “avian influenza virus mutagenesis,” has been used here to identify potentially dangerous research that might fall under the Experiments of Concern (EoC). To focus on the most recent research, only research over the last two years (September 1, 2013 through August 29, 2015) published Pub Med abstracts were read. Thirty-five potential EoC were identified in 136 abstracts for this single search term. Many of the 136 abstracts (136-35=101) described research that did not constitute EoC; for the most part, they did not employ live viruses.
For each of the 35 abstracts that seemed to describe EoC, parts of the full research papers were read to confirm their EoC status. Since I have only a modest grasp of molecular virology, I may have labeled a few that are not EoC, and I may have missed a few that are EoC.
The actual number of EoC research being carried out today is likely much greater than 35 because of the following:
• Only a single avian influenza search term was used; other influenza search terms would yield additional EoC. In particular, viruses that have already caused pandemics such at the 2009 H1N1 virus.
• Expanding the search back to 2012, and even before that, would yield more EoC.
• There are surely some EoC that are not yet published.
• Search terms involving other pathogens such as SARS, MERS and Ebola would yield more EoC.
A summary of the 35 EoC found from the search is provided in Table 1. Titles and citations for the reference numbers are in the reference list at the end.
The 35 published research listed in the Table are described briefly below. The descriptions are a combination of quotes from the Pub Med abstracts and full papers, often paraphrased to make them readily understandable with regard to EoC. The numbers, 1 through 35, at the beginning of each entry below correspond to the numbered reference citations at the end of this document. The bold-face [italicized] highlighted descriptions are the greatest concern in my opinion because the mutated viruses are often more pathogenic than the wild-type strains and are potentially airborne transmissible from human to human.
1. Recombinant influenza viruses were made that have single or double substitutions in neuraminidase N3, N7 and N9 subtypes in a background of an H1N1 vaccine strain. N3, N7 and N9 subtypes have caused human infections. The research discovered resistance to neuraminidase inhibitors in some strains. [Comment: Mutagenesis of vaccine strains are not of the highest concern, unless there is reason to believe that the mutagenesis could make the strain virulent.]
2. Avian H6N1 virus was adapted to human receptor-binding. Receptor-binding was analyzed using isolated H6 proteins. Binding was confirmed using two avian and one human-derived H6N1 recombinant viruses. The research found two HA substitutions important to acquire the human receptor-binding. [Comment: Only one case of human H6N1 infection has been reported to date. Could increasing receptor binding in humans lead to more human cases?]
3. Site-directed mutagenesis was used to generate different patterns of stem glycans on the HA protein of an HPAI H5N1. The results indicated that some glycans were dispensable for the generation of replication-competent influenza viruses. Some combinations of glycans led to a significant decrease of the growth rates of the mutant viruses in animal cells in comparison to wild type virus. Furthermore, most of the mutant viruses were more sensitive to neutralizing antibodies than the WT virus. [Comment: Could researchers predict results in advance? These are experiments that should be proactively reviewed, as some mutations could have increased virulence or avoided existing vaccines. The outcome is, however, reassuring]
4. Variant H5N1 viruses with five mutations in the HA gene were made. The research indicated that targeted mutation in the HA may be effectively used as a tool to develop broadly reactive influenza vaccines to cope with the continuous antigenic evolution of viruses. [Comment: Could researchers predict results in advance? These are experiments that should be proactively reviewed, as some mutations could have increased virulence or avoid existing vaccines. As viral mutant population sizes are huge, the probability of finding an adaptive mutation is pretty large for RNA viruses.]
5. The research found three mutations in HA, N and PB2 proteins that after four passages conferred high virulence to H9N2 virus in mice. Adaption in mice enhanced the viral polymerase activity and receptorbinding ability, which resulted in a virulent phenotype in mice but not a transmissible phenotype to guinea pigs. [Comment: This additional guinea pig experiment was useful to reduce concern or fear over increased host range.]
6. Mutations made in the PA protein enhanced HPAI H5N1 virus growth capability in human lung cells and increased pathogenicity in mice, suggesting that they contribute to adaptation to mammalian hosts.
7. Mutants made with substitutions in the hemagglutinin of a strain of 2009 H1N1 pandemic influenza virus revealed that single substitutions affecting the loop adjacent to the receptor binding site caused escape from ferret and human antibodies elicited after the 2009 H1N1 pandemic influenza virus infection. The majority of these substitutions resulted in similar or increased replication efficiency in vitro compared to that of the virus carrying the wild-type hemagglutinin. However, none of the substitutions was sufficient for escape from the antibodies in sera from individuals that experienced both seasonal and pandemic H1N1 virus infections. [Comment: This is the virus that infected 25% of the world population world-wide in 2009 and killed thousands of people. Any experiment that increases replication efficiency or escapes antibodies should not be carried out in BSL2.]
8. Mutant HPAI H5N1 viruses made with loss of two HA protein glycosylation sites showed increased pathogenicity, systemic spread and pulmonary inflammation in mice compared to the wild-type H5N1 virus.
9. Two mouse-adapted variants of wild-type avian H7N9 made by independent serial passages in mice confer enhanced virulence in mammals. [Comment: This virus has infected and caused fatalities in humans from direct contact with poultry. It would have been informative if the researchers had carried out a single ferret to ferret transmission experiment to see if this mouse-passaged virus has increased host range and virulence in a species (ferrets) that is perhaps a model for humans.]
10. Mouse-adapted PB2 gene reassortants with a phenylalanine-to-leucine mutation contributes to enhanced polymerase activity, enhanced replication, pathogenicity of H9N2 in mice, increased virulence of H5N1 and 2009 pandemic H1N1. [Comment: Could increasing virulence in the 2009 pandemic flu cause a new outbreak among humans?]
11. The introduction of an arginine residue into PA of HPAI H5N1 significantly increased the viral polymerase activity in mammalian cells and its virulence and pathogenicity in mice.
12. A substitution in the PB2 protein and a substitution in the PA protein enhance virulence and expand the tropism of H6N1 virus in mice. [Comment: Only one case of human H6N1 infection has been reported to date. Could increasing virulence and tropism in humans lead to more human cases?]
13. Introduction of a single substitution into PB1 polymerase of an HPAI H5N1 increased both polymerase activity in chicken cells and the pathogenicity of the recombinant viruses in chickens. [Comment: This translates to humans.]
14. A nonpathogenic duck-origin H9N2 virus was serial-passaged in mouse lungs. Increased virulence was detectable after five passages, and a highly pathogenic mouse-adapted strain was obtained after 18 passages. There were eight amino-acid substitutions in six viral proteins. [Comment: Since serial passage was in lungs, this kind of research could lead to airborne transmission. A single ferret to ferret passage experiment should have been carried out to see if airborne transmission was achieved.]
15. A deletion in the NS segment of a duck-origin avian H1N1 virus showed both increased replication potential and an increased pathogenicity in chicken embryonated eggs and in a chicken lung epithelial cell line.
16. Mutants created in the PB2 subunit identified critical residues required for general polymerase function and specific residues preferentially required in human but not avian cells. [Comment: It is unclear what virus was used in the study. It may have been PB2 mutants reassorted into A/WSN/1933 H1N1 virus. A/WSN/1933 is a derivative of 1918 flu virus and is not around today. This is a mouse brain adapted virus so not a threat.]
17. Five substitutions proved to be sufficient to retain the airborne-transmissible phenotype of HPAI H5N1. [Comment: A large number of substitution experiments on an airborne transmissible, deadly virus were carried out in this study, and a large number of nose and throat swabs and blood samples were taken, all increasing significantly the likelihood of an LAI. This is follow-up research from the Fouchier lab.]
18. An H7N9 virus from a fatal case was used as the recombination background to study the contribution of the E627K mutation in PB2 and of other mutations to the pathogenicity of H7N9 virus infection in mammals. All the mutant viruses generated were likely to be loss-of-function mutants with regard to pathogenicity, compared to the wild-type H7N9. [Comment: The research appears to yield less pathogenic H7N9. Nonetheless, it is not possible to predict pathogenicity at the outset of the experiments. Since the background virus is a fatal case; proactively, the generated viruses could have been more virulent humans. It would have been informative if the researchers had carried out a single ferret to ferret transmission experiment to see if this virus was more virulent in ferrets, the model for human lung.]
19. Potentially mammalian adapting amino acids were converted individually and in combination to their avian virus-type counterparts in a H7N9 virus. Several mutants were slightly more virulent in mice than the wild-type A(H7N9) virus and exhibited increased polymerase activity in human cells.
20. A single “consensus” PB2 mutation common to swine and the 2009 H1N1 pandemic virus increased pathogenicity. Mutant virus prepared by recombination of a 2009 H1N1 pandemic virus with a segment containing the single PB2 mutation significantly enhanced polymerase activity in mammalian cells. Also, the virus exhibited increased growth properties and induced significant weight loss in a mouse model compared to the wild type. [Comments: This more pathogenic virus could win the battle with the immune system, so cause significant illness.]
21. Reduced sensitivities to oseltamivir were observed in three mutant H1N1 2009 pandemic viruses. A double mutant showed a large increase of IC-50 for the drug Oseltamivir from 0.7 nM for WT to 4,000 nM for the double mutant, a 5,700-fold difference [Comment: Such a large increase in IC-50 would almost certainly make the drug unusable in humans.]
[22-27 MISSING]
28. A non-pathogenic avian H5N2 was adapted to mice by lung-to-lung passage. Also, the reverse genetics-derived influenza virus containing the HA and NA genes of an HPAI H5N1 in the genetic background of a high-growth H1N1 vaccine strain was obtained. Antibody escape mutants using these two viruses were obtained. Monitoring of effects of HA mutations found in H5 segment escape mutants is essential for accurate prediction of mutants with pandemic potential. [Comment: While H5N2 does not appear to have caused any human infections, adapting it to mice by lung to lung passage could have made it virulent in humans and even airborne transmissible.]
29. Influenza A viruses circulating in humans from ∼1950 to ∼1987 featured a nonstructural (NS1) protein with a C-terminal amino acid extension present in the H3N2 1968 pandemic flu virus. This research deleted the NS1 extension in the H3N2 in order to compare the wild type H3N2 with the virus with the NS1 deletion. The replication kinetics of the wild-type H3N2 and the deletion mutant were indistinguishable in most experimental systems. However, wild-type virus out-competed the mutant during mixed infections, suggesting that the NS1 extension conferred minor growth advantages. [Comment: The resurrection/rescue of an historical pandemic virus is potentially as dangerous as a lab-made PPP if it escapes from the laboratory, provided that the virus employed is identical to or very close to the 1968 pandemic strain.]
[30 MISSING]
31. A particular point mutation in the PB2 protein of HPAI H5N1 virus, PB2 627K, has been identified as a virulence and host range determinant for infection of mammals, and is present in strains capable of airborne transmission. This mutation in the PB2 gene appeared from day 4 and 5 along the respiratory tracts of mice inoculated intranasally and was complete by day 6 post-inoculation. The mutation correlated with efficient replication of the virus in mice. [Comment: This kind of experiment may be on a path to an airborne transmissible strain.]
32. This research focused on the particular PB2 point mutation in Reference 31, just above. Viruses constructed by reverse genetics were made to contain converse PB2 627K/E mutations in a Eurasian HPAI H5N1 virus and, for comparison, a historical pre-Asian HPAI H5N1 virus that naturally bears PB2 627E. Effects on viral fitness were observed in in vitro or in vivo experiments. Results suggest that the PB2 627K mutation supports viral fitness in Eurasian-lineage viruses; in contrast, the mutation carries a significant fitness cost in a historical pre-Asian virus.
[33 MISSING]
34. Influenza virus entry is mediated by the acidic-pH-induced activation of HA protein. This research investigated how a decrease in the HA activation pH influences the properties of highly pathogenic H5N1 influenza virus in mammalian hosts. Viruses containing either wild-type HA or an acid-stabilizing point mutation were prepared. Wild-type and viruses with the mutation promoted similar levels of morbidity and mortality in mice and ferrets. The mutation was found to enhance the growth of an H5N1 influenza virus in the mammalian upper respiratory tract, and yet it was insufficient to enable contact transmission in ferrets. Neither virus transmitted efficiently to naive contact cage-mate ferrets. [Comment: It is fortunate that contact transmission was not found.]
35. The research focused on an antigenic cluster associated with a natural single hemagglutinin (HA) substitution that occurred between 1992 and 1995 in the H3N2 virus. Reverse-genetics experiments demonstrated that the HA mutation increases viral receptor binding avidity. The mutation does not prevent antibody binding; rather, viruses possessing this mutation escape antisera simply because the virus attaches to cells more efficiently. [Comment: The H3N2 virus has caused human infections when transmitted from swine. In a 2012 small outbreak, there was no evidence of community transmission. Nonetheless, the virus is an immune escape strain.]
While the search term was not designed to pick up the 2009 human pandemic H1N1 virus, it did pick up a few experiments involving mutagenesis of that strain. While some of this research is carried out at BSL2, it could be classified as research of great concern because that virus is airborne transmissible.
For research involving mutagenesis of vaccine strains, biosafety level was generally not reported. It is assumed that it is BSL2, as vaccine strains are attenuated or inactivated viruses. One concern is that some mutagenesis research could make a vaccine strain virulent. Researchers should be prepared to argue for the safety of their particular proposed vaccine-strain mutagenesis research to defend the lower BSL2 containment.
Several of the EoC (references 7, 10, 14, 17, 20, 21, 28, 29) are lab-made potentially dangerous influenza viruses that could spread from human to human by the airborne route.
Proactive review at the local, national, or international level that considers risk and value (benefits) should be considered before allowing any mutagenesis and related research that might result in Experiments of Concern to go forward, and under what conditions.
Conclusion
Research that employs, makes, or could make airborne transmissible strains is of the greatest concern. All this research should be subject to proactive international review and oversight. There is an urgent need for a binding international process. While the NSABB mandate is likely restricted to NIH-funded research or perhaps any research in the United States, it behooves the NSABB to urge the State Department to seek a binding international agreement for proactive review and oversight of potential pandemic research.
I would like to thank Simon Wain-Hobson for comments and insights.
REFERENCES
1. Song MS(1), Marathe BM(2), Kumar G(3), Wong SS(2), Rubrum A(2), Zanin M(2), Choi YK(4), Webster RG(2), Govorkova EA(2), Webby RJ(5). Unique determinants of neuraminidase inhibitor resistance among N3, N7, and N9 avian influenza viruses. JVI.01514-15. [2015, Epub ahead of print]
2. Wang F(1), Qi J(2), Bi Y(2), Zhang W(2), Wang M(1), Zhang B(3), Wang M(4), Liu J(4), Yan J(2), Shi Y(5), Gao GF(6). Adaptation of avian influenza A (H6N1) virus from avian to human receptor-binding preference. EMBO J. 2015 Jun 12;34(12):1661-73. doi: 10.15252/embj.201590960.
3. Zhang X(1), Chen S(1), Yang D(1), Wang X(1), Zhu J(1), Peng D(2), Liu X(1). Role of stem glycans attached to haemagglutinin in the biological characteristics of H5N1 avian influenza virus. J Gen Virol. 2015 Jun;96(Pt 6):1248-57. doi: 10.1099/vir.0.000082.
4. Ibrahim M(1), Sultan HA(2), Razik AG(2), Kang KI(3), Arafa AS(4), Shehata AA(2), Saif YM(5), Lee CW(6). Development of broadly reactive H5N1 vaccine against different Egyptian H5N1 viruses. Vaccine. 2015 May 28;33(23):2670-7. doi: 10.1016/j.vaccine.2015.04.023.
5. Sang X(1), Wang A, Chai T, He X, Ding J, Gao X, Li Y, Zhang K, Ren Z, Li L, Yu Z, Wang T, Feng N, Zheng X, Wang H, Zhao Y, Yang S, Gao Y, Xia X. Rapid emergence of a PB2-E627K substitution confers a virulent phenotype to an H9N2 avian influenza virus during adoption in mice. Arch Virol. 2015 May;160(5):1267-77. doi: 10.1007/s00705-015-2383-5.
6. Yamaji R(1), Yamada S(2), Le MQ(3), Ito M(1), Sakai-Tagawa Y(1), Kawaoka Y(4). Mammalian adaptive mutations of the PA protein of highly pathogenic avian H5N1 influenza virus. J Virol. 2015 Apr;89(8):4117-25. doi: 10.1128/JVI.03532-14.
7. Koel BF(1), Mögling R(2), Chutinimitkul S(1), Fraaij PL(3), Burke DF(4), van der Vliet S(1), de Wit E(1), Bestebroer TM(1), Rimmelzwaan GF(1), Osterhaus AD(1), Smith DJ(5), Fouchier RA(1), de Graaf M(6). Identification of amino acid substitutions supporting antigenic change of influenza A(H1N1)pdm09 viruses. J Virol. 2015 Apr;89(7):3763-75. doi: 10.1128/JVI.02962-14.
8. Zhang X(1), Chen S(1), Jiang Y(1), Huang K(1), Huang J(1), Yang D(1), Zhu J(1), Zhu Y(1), Shi S(1), Peng D(2), Liu X(1). Hemagglutinin glycosylation modulates the pathogenicity and antigenicity of the H5N1 avian influenza virus. Vet Microbiol. 2015 Feb 25;175(2-4):244-56. doi: 10.1016/j.vetmic.2014.12.011.
9. Yu Z(1), Sun W(2), Li X(3), Chen Q(4), Chai H(5), Gao X(2), Guo J(2), Zhang K(2), Wang T(2), Feng N(2), Zheng X(2), Wang H(2), Zhao Y(2), Qin C(6), Huang G(2), Yang S(2), Hua Y(5), Zhang X(7), Gao Y(8), Xia X(9). Adaptive amino acid substitutions enhance the virulence of a reassortant H7N1 avian influenza virus isolated from wild waterfowl in mice. Virology. 2015 Feb;476:233-9. doi: 10.1016/j.virol.2014.11.031.
10. Liu Q(1), Huang J(1), Chen Y(1), Chen H(1), Li Q(1), He L(1), Hao X(1), Liu J(1), Gu M(1), Hu J(1), Wang X(1), Hu S(1), Liu X(1), Liu X(2). Virulence determinants in the PB2 gene of a mouse-adapted H9N2 virus. J Virol. 2015 Jan;89(1):877-82. doi: 10.1128/JVI.01775-14.
11. Fan S(1), Hatta M(1), Kim JH(1), Le MQ(2), Neumann G(1), Kawaoka Y(3). Amino acid changes in the influenza A virus PA protein that attenuate avian H5N1 viruses in mammals. J Virol. 2014 Dec;88(23):13737-46. doi: 10.1128/JVI.01081-14.
12. Cheng K(1), Yu Z(2), Chai H(3), Sun W(4), Xin Y(4), Zhang Q(5), Huang J(4), Zhang K(4), Li X(4), Yang S(4), Wang T(4), Zheng X(4), Wang H(4), Qin C(6), Qian J(4), Chen H(5), Hua Y(7), Gao Y(8), Xia X(9). PB2-E627K and PA-T97I substitutions enhance polymerase activity and confer a virulent phenotype to an H6N1 avian influenza virus in mice. Virology. 2014 Nov;468-470:207-13. doi: 10.1016/j.virol.2014.08.010.
13. Suzuki Y(1), Uchida Y(2), Tanikawa T(1), Maeda N(1), Takemae N(2), Saito T(3). Amino acid substitutions in PB1 of avian influenza viruses influence pathogenicity and transmissibility in chickens. J Virol. 2014 Oct;88(19):11130-9. doi: 10.1128/JVI.01564-14.
14. Liu Q(1), Chen H, Huang J, Chen Y, Gu M, Wang X, Hu S, Liu X, Liu X. A nonpathogenic duck-origin H9N2 influenza A virus adapts to high pathogenicity in mice. Arch Virol. 2014 Sep;159(9):2243-52. doi: 10.1007/s00705-014-2062-y.
15. Trapp S(1), Soubieux D(2), Marty H(1), Esnault E(1), Hoffmann TW(3), Chandenier M(3), Lion A(1), Kut E(1), Quéré P(1), Larcher T(4), Ledevin M(4), Munier S(5), Naffakh N(5), Marc D(6). Shortening the unstructured, interdomain region of the non-structural protein NS1 of an avian H1N1 influenza virus increases its replication and pathogenicity in chickens. J Gen Virol. 2014 Jun;95(Pt 6):1233-43. doi: 10.1099/vir.0.063776-0.
16. Kirui J(1), Bucci MD, Poole DS, Mehle A. Conserved features of the PB2 627 domain impact influenza virus polymerase function and replication. J Virol. 2014 Jun;88(11):5977-86. doi: 10.1128/JVI.00508-14.
17. Linster M(1), van Boheemen S(1), de Graaf M(1), Schrauwen EJ(1), Lexmond P(1), Mänz B(1), Bestebroer TM(1), Baumann J(2), van Riel D(1), Rimmelzwaan GF(1), Osterhaus AD(1), Matrosovich M(2), Fouchier RA(3), Herfst S(1). Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell. 2014 Apr 10;157(2):329-39. doi: 10.1016/j.cell.2014.02.040.
18. Mok CK(1), Lee HH, Lestra M, Nicholls JM, Chan MC, Sia SF, Zhu H, Poon LL, Guan Y, Peiris JS. Amino acid substitutions in polymerase basic protein 2 gene contribute to the pathogenicity of the novel A/H7N9 influenza virus in mammalian hosts. J Virol. 2014 Mar;88(6):3568-76. doi: 10.1128/JVI.02740- 13.
19. Yamayoshi S(1), Yamada S, Fukuyama S, Murakami S, Zhao D, Uraki R, Watanabe T, Tomita Y, Macken C, Neumann G, Kawaoka Y. Virulence-affecting amino acid changes in the PA protein of H7N9 influenza A viruses. J Virol. 2014 Mar;88(6):3127-34. doi: 10.1128/JVI.03155-13.
20. Zhao Z(1), Yi C, Zhao L, Wang S, Zhou L, Hu Y, Zou W, Chen H, Jin M. PB2-588I enhances 2009 H1N1 pandemic influenza virus virulence by increasing viral replication and exacerbating PB2 inhibition of beta interferon expression. J Virol. 2014 Feb;88(4):2260-7. doi: 10.1128/JVI.03024-13.
21. Huang L(1), Cao Y, Zhou J, Qin K, Zhu W, Zhu Y, Yang L, Wang D, Wei H, Shu Y. A conformational restriction in the influenza A virus neuraminidase binding site by R152 results in a combinational effect of I222T and H274Y on oseltamivir resistance.
22. Pérez-Cidoncha M(1), Killip MJ(2), Asensio VJ(3), Fernández Y(1), Bengoechea JA(4), Randall RE(2), Ortín J(1). Generation of replication-proficient influenza virus NS1 point mutants with interferonhyperinducer phenotype. PLoS One. 2014 Jun 2;9(6):e98668. doi: 10.1371/journal.pone.0098668.
23. Sitaras I(1), Kalthoff D(2), Beer M(2), Peeters B(3), de Jong MC(4). Immune escape mutants of Highly Pathogenic Avian Influenza H5N1 selected using polyclonal sera: identification of key amino acids in the HA protein. PLoS One. 2014 Feb 25;9(2):e84628. doi: 10.1371/journal.pone.0084628.
24. Heaton NS(1), Sachs D, Chen CJ, Hai R, Palese P. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):20248-53. doi: 10.1073/pnas.1320524110.
25. Tamura D(1), Nguyen HT, Sleeman K, Levine M, Mishin VP, Yang H, Guo Z, Okomo-Adhiambo M, Xu X, Stevens J, Gubareva LV. Cell culture-selected substitutions in influenza A(H3N2) neuraminidase affect drug susceptibility assessment. Antimicrob Agents Chemother. 2013 Dec;57(12):6141-6. doi: 10.1128/AAC.01364-13.
26. Kalthoff D(1), Röhrs S, Höper D, Hoffmann B, Bogs J, Stech J, Beer M. Truncation and sequence shuffling of segment 6 generate replication-competent neuraminidase-negative influenza H5N1 viruses. J Virol. 2013 Dec;87(24):13556-68. doi: 10.1128/JVI.02244-13.
27. Zhu X(1), Guo YH, Jiang T, Wang YD, Chan KH, Li XF, Yu W, McBride R, Paulson JC, Yuen KY, Qin CF, Che XY, Wilson IA. A unique and conserved neutralization epitope in H5N1 influenza viruses identified by an antibody against the A/Goose/Guangdong/1/96 hemagglutinin. J Virol. 2013 Dec;87(23):12619-35. doi: 10.1128/JVI.01577-13.
28. Rudneva IA(1), Timofeeva TA, Ignatieva AV, Shilov AA, Krylov PS, Ilyushina NA, Kaverin NV. Pleiotropic effects of hemagglutinin amino acid substitutions of H5 influenza escape mutants. Virology. 2013 Dec;447(1-2):233-9. doi: 10.1016/j.virol.2013.09.013.
29. Lohrmann F(1), Dijkman R, Stertz S, Thiel V, Haller O, Staeheli P, Kochs G. Emergence of a C-terminal seven-amino-acid elongation of NS1 in around 1950 conferred a minor growth advantage to former seasonal influenza A viruses. J Virol. 2013 Oct;87(20):11300-3. doi: 10.1128/JVI.01271-13.
30. Myers JL(1), Wetzel KS, Linderman SL, Li Y, Sullivan CB, Hensley SE. Compensatory hemagglutinin mutations alter antigenic properties of influenza viruses. J Virol. 2013 Oct;87(20):11168-72. doi: 10.1128/JVI.01414-13.
31. Min JY(1), Santos C, Fitch A, Twaddle A, Toyoda Y, DePasse JV, Ghedin E, Subbarao K. Mammalian adaptation in the PB2 gene of avian H5N1 influenza virus. J Virol. 2013. Oct;87(19):10884-8. doi: 10.1128/JVI.01016-13.
32. Long JS(1), Howard WA, Núñez A, Moncorgé O, Lycett S, Banks J, Barclay WS. The effect of the PB2 mutation 627K on highly pathogenic H5N1 avian influenza virus is dependent on the virus lineage. J Virol. 2013 Sep;87(18):9983-96. doi: 10.1128/JVI.01399-13.
33. Roberts KL(1), Leser GP, Ma C, Lamb RA. The amphipathic helix of influenza A virus M2 protein is required for filamentous bud formation and scission of filamentous and spherical particles. J Virol. 2013 Sep;87(18):9973-82. doi: 10.1128/JVI.01363-13.
34. Zaraket H(1), Bridges OA, Duan S, Baranovich T, Yoon SW, Reed ML, Salomon R, Webby RJ, Webster RG, Russell CJ. Increased Acid Stability of the Hemagglutinin Protein Enhances H5N1 Influenza Virus Growth in the Upper Respiratory Tract but Is Insufficient for Transmission in Ferrets. J Virol. 2013 Sep;87(17):9911-22. doi: 10.1128/JVI.01175-13.
35. Li Y(1), Bostick DL, Sullivan CB, Myers JL, Griesemer SB, Stgeorge K, Plotkin JB, Hensley SE. Single hemagglutinin mutations that alter both antigenicity and receptor binding avidity influence influenza virus antigenic clustering. J Virol. 2013 Sep;87(17):9904-10. doi: 10.1128/JVI.01023-13.
by Lynn C. Klotz, Ph.D., Senior Science Fellow and member of the Scientist Working Group on Biological and Chemical Weapons Center for Arms Control and Non-proliferation, Washington, DC, USA
September 2015
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT
YOU ARE REQUIRED TO READ THE COPYRIGHT NOTICE AT THIS LINK BEFORE YOU READ THE FOLLOWING WORK, THAT IS AVAILABLE SOLELY FOR PRIVATE STUDY, SCHOLARSHIP OR RESEARCH PURSUANT TO 17 U.S.C. SECTION 107 AND 108. IN THE EVENT THAT THE LIBRARY DETERMINES THAT UNLAWFUL COPYING OF THIS WORK HAS OCCURRED, THE LIBRARY HAS THE RIGHT TO BLOCK THE I.P. ADDRESS AT WHICH THE UNLAWFUL COPYING APPEARED TO HAVE OCCURRED. THANK YOU FOR RESPECTING THE RIGHTS OF COPYRIGHT OWNERS.
To: The NSABB Board (in advance of the September 28, 2015 meeting)
From: Lynn C. Klotz, PhD, Senior Science Fellow and member of the Scientist Working Group on Biological and Chemical Weapons Center for Arms Control and Non-proliferation, Washington, DC, USA
Home contact Information: 5 Duley Street, Gloucester MA 01930, 978-281-6015, [email protected]
The following document is a first draft of a literature-research project that started in the summer of 2015. The project is in an early stage, but has gone far enough to make its point: There is an urgent need for international proactive oversight of influenza research that might increase the pathogenicity of influenza viruses. Some of this gain-of-function research may create lab-made potential pandemic influenza viruses.
Even if the probability is small for an escape from a lab in a single year for such a virus, the fact that there are a large number of research projects underway throughout the world, projects that will be conducted for many years, the overall probability of escape from at least one lab is uncomfortably high.
The Potential Pandemic Influenza Research Enterprise
In a recent Letter to the Editor titled Danger of Potential-Pandemic-Pathogen Research Enterprises (http://intl-mbio.asm.org/content/6/3/e00815-15.full), I argued that there are likely many labs throughout the world, many not funded by the NIH, that are developing mammal-contagious influenza viruses. Research that makes avian, mammalian, or human influenza viruses more virulent, increases their transmissibility, alters their host range, or evades countermeasures is potentially dangerous and may create potential pandemic pathogens.
Influenza viruses are more likely to fuel an uncontrollable outbreak because of their long history of doing just that. This kind of research got considerable attention in 2011 when Professor Ron Fouchier announced that his laboratory had made the H5N1 highly pathogenic avian influenza virus (HPAI) airborne transmissible by respiratory aerosols from ferret to ferret.
In the context of this analysis of recent publications reported in Pub Med (references (1) through (35)), the larger category of Experiments of Concern (EoC) is used as a guide to look for potentially dangerous research. In 2004, the National Academy of Sciences published a report Biotechnology Research in an Age of Terrorism (http://www.nap.edu/catalog/10827.html). The so-called Fink Committee that produced the report was asked to “consider ways to minimize threats from biological warfare and bioterrorism without hindering the progress of biotechnology, which is essential for the health of the nation.” The committee recommended that the “Department of Health and Human Services…create a review system for seven classes of experiments (the Experiments of Concern) involving microbial agents that raise concerns about their potential for misuse.” Specifically, the EoC are:
“1. Would demonstrate how to render a vaccine ineffective. This would apply to both human and animal vaccines…
2. Would confer resistance to therapeutically useful antibiotics or antiviral agents. This would apply to therapeutic agents that are used to control disease agents in humans, animals or crops…
3. Would enhance the virulence of a pathogen or render a non-pathogen virulent. This would apply to plant, animal, and human pathogens…
4. Would increase transmissibility of a pathogen. This would include enhancing transmission within or between species. Altering vector competence to enhance disease transmission would also fall into this class.
5. Would alter the host range of a pathogen. This would include making non zoonotics into zoonotic agents. Altering the tropism of viruses would fit into this class.
6. Would enable the evasion of diagnostic/detection modalities. This could include microencapsulation to avoid antibody-based detection and/or the alteration of gene sequences to avoid detection by established molecular methods.
7. Would enable the weaponization of a biological agent or toxin. This would include the environmental stabilization of pathogens.”
These seven classes of experiments “will require review and discussion by informed members of the scientific and medical community before they are undertaken [proactive oversight] or, if carried out, before they are published in full detail.” For experiments making deadly avian influenza viruses airborne transmissible, many scientists think they should not be carried out at all.
An excellent system for reviewing potentially dangerous experiments, Controlling Dangerous Pathogens: A Prototype Protective Oversight System, was developed in 2007 by The Center for International and Security Studies at Maryland (http://drum.lib.umd.edu/bitstream/1903/ ... ograph.pdf). It recommends a tiered review, from most to least dangerous research. Paraphrased from the Maryland paper:
International Oversight: Activities of Extreme Concern –- An international body would be charged with approving and monitoring all research projects of extreme concern. That authority would be narrowly focused only on those ... that could put an appreciable fraction of the human species at risk, such as research with potential pandemic pathogens.
National Oversight: Activities of Moderate Concern -– National oversight bodies would be responsible for research activity of moderate concern, such as work with anthrax and other agents already identified as having biological weapons potential.
Local Oversight: Activities of Potential Concern—This “encompasses those activities that may increase the destructive potential of biological agents that otherwise would not be considered a threat.
No oversight: All other research
In my opinion, there should be two levels of local oversight. The first level is the currently employed Institutional Biosafety Committee (IBC), and the second is an outside committee. There is concern that IBCs will simply rubber-stamp research proposals from labs in their own institution, so I suggest proactive oversight by a committee outside the institution (perhaps at the state level in the US) for experiments of concern on influenza viruses that do not carry an immediate threat of an outbreak from an escape from the laboratory (e.g., vaccine viruses and other attenuated and inactivated viruses).
Because of the potential for some strains of lab-made influenza viruses to cause international outbreaks, research mutagenizing these viruses that could result in increased pathogenicity (gain of function) should be subject to external oversight. At present, there is little national and no international proactive oversight with any authority to guide or ban experiments. See for instance: Gronvall GK, Rozo M. Synopsis of Biological Safety and Security Arrangements. UPMC Center for Health Security.July 2015. Available at http://www.upmchealthsecurity.org/ourwo ... rangements.
The literature analysis
To date, only one general Pub Med search term, “avian influenza virus mutagenesis,” has been used here to identify potentially dangerous research that might fall under the Experiments of Concern (EoC). To focus on the most recent research, only research over the last two years (September 1, 2013 through August 29, 2015) published Pub Med abstracts were read. Thirty-five potential EoC were identified in 136 abstracts for this single search term. Many of the 136 abstracts (136-35=101) described research that did not constitute EoC; for the most part, they did not employ live viruses.
For each of the 35 abstracts that seemed to describe EoC, parts of the full research papers were read to confirm their EoC status. Since I have only a modest grasp of molecular virology, I may have labeled a few that are not EoC, and I may have missed a few that are EoC.
The actual number of EoC research being carried out today is likely much greater than 35 because of the following:
• Only a single avian influenza search term was used; other influenza search terms would yield additional EoC. In particular, viruses that have already caused pandemics such at the 2009 H1N1 virus.
• Expanding the search back to 2012, and even before that, would yield more EoC.
• There are surely some EoC that are not yet published.
• Search terms involving other pathogens such as SARS, MERS and Ebola would yield more EoC.
A summary of the 35 EoC found from the search is provided in Table 1. Titles and citations for the reference numbers are in the reference list at the end.
Reference No. / Countries of Authors / Viruses / Biosafety Level / EOC Category
1 / USA, Korea / H1N1 vaccine strain / ? / 2
2 / China / Avian, human H6N1 / BSL3 / 5
3 / China / H5N1 HPAI / not reported / 1, 3
4 / USA, Egypt / H5N1 HPAI / ? / 1, 3
5 China / H9N2 avian / BSL3 / 3, 5
6 / Japan, USA / H5N1 HPAI / BSL3 / 3, 5
7 / Netherlands, UK / H1N1 2009 / BSL2 / 1, 3
8 / China / H5N1 HPAI / Not reported / 3
9 / China / H7N1 avian / BSL3 / 3, 5
10 / China H9N2, H1N1 2009, H5N1 HPAI / BSL3, BSL3+ / 3
11 / USA, Japan / H5N1 HPAI / BSL3 / 3
12 / China / H6N1 avian / ?? / 3, 5
13 / Japan, Thailand / H5N1 HPAI / BSL3 / 3
14 / China / H9N2 duck / ABSL3+ / 3, 5
15 / France / avian H1N1 / BSL3+ / 3, 5
16 / USA / A/WSN/1933 H1N1 / likely BSL2 / 5
17 / Netherlands / airborne trans H5N1 HPAI animal / BSL3+ / 3, 4
18 / China / H7N9 HPAI / ABSL3 / 3
19 / Japan / H7N9 HPAI / BSL3+ / 3
20 / China / H1N1 2009 pandemic / not reported, BSL2? / 3
21 / China / H1N1 2009 pandemic / not reported, BSL2? / 2
22 / Spain, UK / influenza A vaccine strains / assume BSL2 / 3
23 / Netherl., Germany / HPAI H5N1 / BSL3+ / 1
24 / USA ; H1N1 vaccine strain / assume BSL2 / 1
25 / USA / H3N2 / BSL2? / 2
26 / Germany / HPAI H5N1 / BSL3+ / 1
27 / China, USA / HPAI H5N1 / BSL3, ABSL3 / 2
28 / Russia / nonpath H5N2, HPAI H5N1 / not reported, BSL2? / 1
29 / Germany / 1968 pandemic H3N2 / not reported, BSL2? / 3?
30 / USA / H1N1 vaccine strain / not reported, BSL2? / 1
31 / USA / HPAI H5N1 / BSL3 / 3, 5
32 / UK HPAI H5N1 / BSL3 / 3, 5
33 / USA / H3N2, H1N1 / not reported, BSL2? / 3?
34 / USA / HPAI H5N1 / ABSL3+ / 3, 4
35 / USA / human H3N2, HPAI H5N1 / ABSL3+ / 1
Table 1: The 35 EoC. The boldface in the Countries of Authors column indicates the country where the BSL2, BSL3 research was performed. Much of that research is being carried out in Asia, particularly China.
The 35 published research listed in the Table are described briefly below. The descriptions are a combination of quotes from the Pub Med abstracts and full papers, often paraphrased to make them readily understandable with regard to EoC. The numbers, 1 through 35, at the beginning of each entry below correspond to the numbered reference citations at the end of this document. The bold-face [italicized] highlighted descriptions are the greatest concern in my opinion because the mutated viruses are often more pathogenic than the wild-type strains and are potentially airborne transmissible from human to human.
1. Recombinant influenza viruses were made that have single or double substitutions in neuraminidase N3, N7 and N9 subtypes in a background of an H1N1 vaccine strain. N3, N7 and N9 subtypes have caused human infections. The research discovered resistance to neuraminidase inhibitors in some strains. [Comment: Mutagenesis of vaccine strains are not of the highest concern, unless there is reason to believe that the mutagenesis could make the strain virulent.]
2. Avian H6N1 virus was adapted to human receptor-binding. Receptor-binding was analyzed using isolated H6 proteins. Binding was confirmed using two avian and one human-derived H6N1 recombinant viruses. The research found two HA substitutions important to acquire the human receptor-binding. [Comment: Only one case of human H6N1 infection has been reported to date. Could increasing receptor binding in humans lead to more human cases?]
3. Site-directed mutagenesis was used to generate different patterns of stem glycans on the HA protein of an HPAI H5N1. The results indicated that some glycans were dispensable for the generation of replication-competent influenza viruses. Some combinations of glycans led to a significant decrease of the growth rates of the mutant viruses in animal cells in comparison to wild type virus. Furthermore, most of the mutant viruses were more sensitive to neutralizing antibodies than the WT virus. [Comment: Could researchers predict results in advance? These are experiments that should be proactively reviewed, as some mutations could have increased virulence or avoided existing vaccines. The outcome is, however, reassuring]
4. Variant H5N1 viruses with five mutations in the HA gene were made. The research indicated that targeted mutation in the HA may be effectively used as a tool to develop broadly reactive influenza vaccines to cope with the continuous antigenic evolution of viruses. [Comment: Could researchers predict results in advance? These are experiments that should be proactively reviewed, as some mutations could have increased virulence or avoid existing vaccines. As viral mutant population sizes are huge, the probability of finding an adaptive mutation is pretty large for RNA viruses.]
5. The research found three mutations in HA, N and PB2 proteins that after four passages conferred high virulence to H9N2 virus in mice. Adaption in mice enhanced the viral polymerase activity and receptorbinding ability, which resulted in a virulent phenotype in mice but not a transmissible phenotype to guinea pigs. [Comment: This additional guinea pig experiment was useful to reduce concern or fear over increased host range.]
6. Mutations made in the PA protein enhanced HPAI H5N1 virus growth capability in human lung cells and increased pathogenicity in mice, suggesting that they contribute to adaptation to mammalian hosts.
7. Mutants made with substitutions in the hemagglutinin of a strain of 2009 H1N1 pandemic influenza virus revealed that single substitutions affecting the loop adjacent to the receptor binding site caused escape from ferret and human antibodies elicited after the 2009 H1N1 pandemic influenza virus infection. The majority of these substitutions resulted in similar or increased replication efficiency in vitro compared to that of the virus carrying the wild-type hemagglutinin. However, none of the substitutions was sufficient for escape from the antibodies in sera from individuals that experienced both seasonal and pandemic H1N1 virus infections. [Comment: This is the virus that infected 25% of the world population world-wide in 2009 and killed thousands of people. Any experiment that increases replication efficiency or escapes antibodies should not be carried out in BSL2.]
8. Mutant HPAI H5N1 viruses made with loss of two HA protein glycosylation sites showed increased pathogenicity, systemic spread and pulmonary inflammation in mice compared to the wild-type H5N1 virus.
9. Two mouse-adapted variants of wild-type avian H7N9 made by independent serial passages in mice confer enhanced virulence in mammals. [Comment: This virus has infected and caused fatalities in humans from direct contact with poultry. It would have been informative if the researchers had carried out a single ferret to ferret transmission experiment to see if this mouse-passaged virus has increased host range and virulence in a species (ferrets) that is perhaps a model for humans.]
10. Mouse-adapted PB2 gene reassortants with a phenylalanine-to-leucine mutation contributes to enhanced polymerase activity, enhanced replication, pathogenicity of H9N2 in mice, increased virulence of H5N1 and 2009 pandemic H1N1. [Comment: Could increasing virulence in the 2009 pandemic flu cause a new outbreak among humans?]
11. The introduction of an arginine residue into PA of HPAI H5N1 significantly increased the viral polymerase activity in mammalian cells and its virulence and pathogenicity in mice.
12. A substitution in the PB2 protein and a substitution in the PA protein enhance virulence and expand the tropism of H6N1 virus in mice. [Comment: Only one case of human H6N1 infection has been reported to date. Could increasing virulence and tropism in humans lead to more human cases?]
13. Introduction of a single substitution into PB1 polymerase of an HPAI H5N1 increased both polymerase activity in chicken cells and the pathogenicity of the recombinant viruses in chickens. [Comment: This translates to humans.]
14. A nonpathogenic duck-origin H9N2 virus was serial-passaged in mouse lungs. Increased virulence was detectable after five passages, and a highly pathogenic mouse-adapted strain was obtained after 18 passages. There were eight amino-acid substitutions in six viral proteins. [Comment: Since serial passage was in lungs, this kind of research could lead to airborne transmission. A single ferret to ferret passage experiment should have been carried out to see if airborne transmission was achieved.]
15. A deletion in the NS segment of a duck-origin avian H1N1 virus showed both increased replication potential and an increased pathogenicity in chicken embryonated eggs and in a chicken lung epithelial cell line.
16. Mutants created in the PB2 subunit identified critical residues required for general polymerase function and specific residues preferentially required in human but not avian cells. [Comment: It is unclear what virus was used in the study. It may have been PB2 mutants reassorted into A/WSN/1933 H1N1 virus. A/WSN/1933 is a derivative of 1918 flu virus and is not around today. This is a mouse brain adapted virus so not a threat.]
17. Five substitutions proved to be sufficient to retain the airborne-transmissible phenotype of HPAI H5N1. [Comment: A large number of substitution experiments on an airborne transmissible, deadly virus were carried out in this study, and a large number of nose and throat swabs and blood samples were taken, all increasing significantly the likelihood of an LAI. This is follow-up research from the Fouchier lab.]
18. An H7N9 virus from a fatal case was used as the recombination background to study the contribution of the E627K mutation in PB2 and of other mutations to the pathogenicity of H7N9 virus infection in mammals. All the mutant viruses generated were likely to be loss-of-function mutants with regard to pathogenicity, compared to the wild-type H7N9. [Comment: The research appears to yield less pathogenic H7N9. Nonetheless, it is not possible to predict pathogenicity at the outset of the experiments. Since the background virus is a fatal case; proactively, the generated viruses could have been more virulent humans. It would have been informative if the researchers had carried out a single ferret to ferret transmission experiment to see if this virus was more virulent in ferrets, the model for human lung.]
19. Potentially mammalian adapting amino acids were converted individually and in combination to their avian virus-type counterparts in a H7N9 virus. Several mutants were slightly more virulent in mice than the wild-type A(H7N9) virus and exhibited increased polymerase activity in human cells.
20. A single “consensus” PB2 mutation common to swine and the 2009 H1N1 pandemic virus increased pathogenicity. Mutant virus prepared by recombination of a 2009 H1N1 pandemic virus with a segment containing the single PB2 mutation significantly enhanced polymerase activity in mammalian cells. Also, the virus exhibited increased growth properties and induced significant weight loss in a mouse model compared to the wild type. [Comments: This more pathogenic virus could win the battle with the immune system, so cause significant illness.]
21. Reduced sensitivities to oseltamivir were observed in three mutant H1N1 2009 pandemic viruses. A double mutant showed a large increase of IC-50 for the drug Oseltamivir from 0.7 nM for WT to 4,000 nM for the double mutant, a 5,700-fold difference [Comment: Such a large increase in IC-50 would almost certainly make the drug unusable in humans.]
[22-27 MISSING]
28. A non-pathogenic avian H5N2 was adapted to mice by lung-to-lung passage. Also, the reverse genetics-derived influenza virus containing the HA and NA genes of an HPAI H5N1 in the genetic background of a high-growth H1N1 vaccine strain was obtained. Antibody escape mutants using these two viruses were obtained. Monitoring of effects of HA mutations found in H5 segment escape mutants is essential for accurate prediction of mutants with pandemic potential. [Comment: While H5N2 does not appear to have caused any human infections, adapting it to mice by lung to lung passage could have made it virulent in humans and even airborne transmissible.]
29. Influenza A viruses circulating in humans from ∼1950 to ∼1987 featured a nonstructural (NS1) protein with a C-terminal amino acid extension present in the H3N2 1968 pandemic flu virus. This research deleted the NS1 extension in the H3N2 in order to compare the wild type H3N2 with the virus with the NS1 deletion. The replication kinetics of the wild-type H3N2 and the deletion mutant were indistinguishable in most experimental systems. However, wild-type virus out-competed the mutant during mixed infections, suggesting that the NS1 extension conferred minor growth advantages. [Comment: The resurrection/rescue of an historical pandemic virus is potentially as dangerous as a lab-made PPP if it escapes from the laboratory, provided that the virus employed is identical to or very close to the 1968 pandemic strain.]
[30 MISSING]
31. A particular point mutation in the PB2 protein of HPAI H5N1 virus, PB2 627K, has been identified as a virulence and host range determinant for infection of mammals, and is present in strains capable of airborne transmission. This mutation in the PB2 gene appeared from day 4 and 5 along the respiratory tracts of mice inoculated intranasally and was complete by day 6 post-inoculation. The mutation correlated with efficient replication of the virus in mice. [Comment: This kind of experiment may be on a path to an airborne transmissible strain.]
32. This research focused on the particular PB2 point mutation in Reference 31, just above. Viruses constructed by reverse genetics were made to contain converse PB2 627K/E mutations in a Eurasian HPAI H5N1 virus and, for comparison, a historical pre-Asian HPAI H5N1 virus that naturally bears PB2 627E. Effects on viral fitness were observed in in vitro or in vivo experiments. Results suggest that the PB2 627K mutation supports viral fitness in Eurasian-lineage viruses; in contrast, the mutation carries a significant fitness cost in a historical pre-Asian virus.
[33 MISSING]
34. Influenza virus entry is mediated by the acidic-pH-induced activation of HA protein. This research investigated how a decrease in the HA activation pH influences the properties of highly pathogenic H5N1 influenza virus in mammalian hosts. Viruses containing either wild-type HA or an acid-stabilizing point mutation were prepared. Wild-type and viruses with the mutation promoted similar levels of morbidity and mortality in mice and ferrets. The mutation was found to enhance the growth of an H5N1 influenza virus in the mammalian upper respiratory tract, and yet it was insufficient to enable contact transmission in ferrets. Neither virus transmitted efficiently to naive contact cage-mate ferrets. [Comment: It is fortunate that contact transmission was not found.]
35. The research focused on an antigenic cluster associated with a natural single hemagglutinin (HA) substitution that occurred between 1992 and 1995 in the H3N2 virus. Reverse-genetics experiments demonstrated that the HA mutation increases viral receptor binding avidity. The mutation does not prevent antibody binding; rather, viruses possessing this mutation escape antisera simply because the virus attaches to cells more efficiently. [Comment: The H3N2 virus has caused human infections when transmitted from swine. In a 2012 small outbreak, there was no evidence of community transmission. Nonetheless, the virus is an immune escape strain.]
While the search term was not designed to pick up the 2009 human pandemic H1N1 virus, it did pick up a few experiments involving mutagenesis of that strain. While some of this research is carried out at BSL2, it could be classified as research of great concern because that virus is airborne transmissible.
For research involving mutagenesis of vaccine strains, biosafety level was generally not reported. It is assumed that it is BSL2, as vaccine strains are attenuated or inactivated viruses. One concern is that some mutagenesis research could make a vaccine strain virulent. Researchers should be prepared to argue for the safety of their particular proposed vaccine-strain mutagenesis research to defend the lower BSL2 containment.
Several of the EoC (references 7, 10, 14, 17, 20, 21, 28, 29) are lab-made potentially dangerous influenza viruses that could spread from human to human by the airborne route.
Proactive review at the local, national, or international level that considers risk and value (benefits) should be considered before allowing any mutagenesis and related research that might result in Experiments of Concern to go forward, and under what conditions.
Conclusion
Research that employs, makes, or could make airborne transmissible strains is of the greatest concern. All this research should be subject to proactive international review and oversight. There is an urgent need for a binding international process. While the NSABB mandate is likely restricted to NIH-funded research or perhaps any research in the United States, it behooves the NSABB to urge the State Department to seek a binding international agreement for proactive review and oversight of potential pandemic research.
I would like to thank Simon Wain-Hobson for comments and insights.
REFERENCES
1. Song MS(1), Marathe BM(2), Kumar G(3), Wong SS(2), Rubrum A(2), Zanin M(2), Choi YK(4), Webster RG(2), Govorkova EA(2), Webby RJ(5). Unique determinants of neuraminidase inhibitor resistance among N3, N7, and N9 avian influenza viruses. JVI.01514-15. [2015, Epub ahead of print]
2. Wang F(1), Qi J(2), Bi Y(2), Zhang W(2), Wang M(1), Zhang B(3), Wang M(4), Liu J(4), Yan J(2), Shi Y(5), Gao GF(6). Adaptation of avian influenza A (H6N1) virus from avian to human receptor-binding preference. EMBO J. 2015 Jun 12;34(12):1661-73. doi: 10.15252/embj.201590960.
3. Zhang X(1), Chen S(1), Yang D(1), Wang X(1), Zhu J(1), Peng D(2), Liu X(1). Role of stem glycans attached to haemagglutinin in the biological characteristics of H5N1 avian influenza virus. J Gen Virol. 2015 Jun;96(Pt 6):1248-57. doi: 10.1099/vir.0.000082.
4. Ibrahim M(1), Sultan HA(2), Razik AG(2), Kang KI(3), Arafa AS(4), Shehata AA(2), Saif YM(5), Lee CW(6). Development of broadly reactive H5N1 vaccine against different Egyptian H5N1 viruses. Vaccine. 2015 May 28;33(23):2670-7. doi: 10.1016/j.vaccine.2015.04.023.
5. Sang X(1), Wang A, Chai T, He X, Ding J, Gao X, Li Y, Zhang K, Ren Z, Li L, Yu Z, Wang T, Feng N, Zheng X, Wang H, Zhao Y, Yang S, Gao Y, Xia X. Rapid emergence of a PB2-E627K substitution confers a virulent phenotype to an H9N2 avian influenza virus during adoption in mice. Arch Virol. 2015 May;160(5):1267-77. doi: 10.1007/s00705-015-2383-5.
6. Yamaji R(1), Yamada S(2), Le MQ(3), Ito M(1), Sakai-Tagawa Y(1), Kawaoka Y(4). Mammalian adaptive mutations of the PA protein of highly pathogenic avian H5N1 influenza virus. J Virol. 2015 Apr;89(8):4117-25. doi: 10.1128/JVI.03532-14.
7. Koel BF(1), Mögling R(2), Chutinimitkul S(1), Fraaij PL(3), Burke DF(4), van der Vliet S(1), de Wit E(1), Bestebroer TM(1), Rimmelzwaan GF(1), Osterhaus AD(1), Smith DJ(5), Fouchier RA(1), de Graaf M(6). Identification of amino acid substitutions supporting antigenic change of influenza A(H1N1)pdm09 viruses. J Virol. 2015 Apr;89(7):3763-75. doi: 10.1128/JVI.02962-14.
8. Zhang X(1), Chen S(1), Jiang Y(1), Huang K(1), Huang J(1), Yang D(1), Zhu J(1), Zhu Y(1), Shi S(1), Peng D(2), Liu X(1). Hemagglutinin glycosylation modulates the pathogenicity and antigenicity of the H5N1 avian influenza virus. Vet Microbiol. 2015 Feb 25;175(2-4):244-56. doi: 10.1016/j.vetmic.2014.12.011.
9. Yu Z(1), Sun W(2), Li X(3), Chen Q(4), Chai H(5), Gao X(2), Guo J(2), Zhang K(2), Wang T(2), Feng N(2), Zheng X(2), Wang H(2), Zhao Y(2), Qin C(6), Huang G(2), Yang S(2), Hua Y(5), Zhang X(7), Gao Y(8), Xia X(9). Adaptive amino acid substitutions enhance the virulence of a reassortant H7N1 avian influenza virus isolated from wild waterfowl in mice. Virology. 2015 Feb;476:233-9. doi: 10.1016/j.virol.2014.11.031.
10. Liu Q(1), Huang J(1), Chen Y(1), Chen H(1), Li Q(1), He L(1), Hao X(1), Liu J(1), Gu M(1), Hu J(1), Wang X(1), Hu S(1), Liu X(1), Liu X(2). Virulence determinants in the PB2 gene of a mouse-adapted H9N2 virus. J Virol. 2015 Jan;89(1):877-82. doi: 10.1128/JVI.01775-14.
11. Fan S(1), Hatta M(1), Kim JH(1), Le MQ(2), Neumann G(1), Kawaoka Y(3). Amino acid changes in the influenza A virus PA protein that attenuate avian H5N1 viruses in mammals. J Virol. 2014 Dec;88(23):13737-46. doi: 10.1128/JVI.01081-14.
12. Cheng K(1), Yu Z(2), Chai H(3), Sun W(4), Xin Y(4), Zhang Q(5), Huang J(4), Zhang K(4), Li X(4), Yang S(4), Wang T(4), Zheng X(4), Wang H(4), Qin C(6), Qian J(4), Chen H(5), Hua Y(7), Gao Y(8), Xia X(9). PB2-E627K and PA-T97I substitutions enhance polymerase activity and confer a virulent phenotype to an H6N1 avian influenza virus in mice. Virology. 2014 Nov;468-470:207-13. doi: 10.1016/j.virol.2014.08.010.
13. Suzuki Y(1), Uchida Y(2), Tanikawa T(1), Maeda N(1), Takemae N(2), Saito T(3). Amino acid substitutions in PB1 of avian influenza viruses influence pathogenicity and transmissibility in chickens. J Virol. 2014 Oct;88(19):11130-9. doi: 10.1128/JVI.01564-14.
14. Liu Q(1), Chen H, Huang J, Chen Y, Gu M, Wang X, Hu S, Liu X, Liu X. A nonpathogenic duck-origin H9N2 influenza A virus adapts to high pathogenicity in mice. Arch Virol. 2014 Sep;159(9):2243-52. doi: 10.1007/s00705-014-2062-y.
15. Trapp S(1), Soubieux D(2), Marty H(1), Esnault E(1), Hoffmann TW(3), Chandenier M(3), Lion A(1), Kut E(1), Quéré P(1), Larcher T(4), Ledevin M(4), Munier S(5), Naffakh N(5), Marc D(6). Shortening the unstructured, interdomain region of the non-structural protein NS1 of an avian H1N1 influenza virus increases its replication and pathogenicity in chickens. J Gen Virol. 2014 Jun;95(Pt 6):1233-43. doi: 10.1099/vir.0.063776-0.
16. Kirui J(1), Bucci MD, Poole DS, Mehle A. Conserved features of the PB2 627 domain impact influenza virus polymerase function and replication. J Virol. 2014 Jun;88(11):5977-86. doi: 10.1128/JVI.00508-14.
17. Linster M(1), van Boheemen S(1), de Graaf M(1), Schrauwen EJ(1), Lexmond P(1), Mänz B(1), Bestebroer TM(1), Baumann J(2), van Riel D(1), Rimmelzwaan GF(1), Osterhaus AD(1), Matrosovich M(2), Fouchier RA(3), Herfst S(1). Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell. 2014 Apr 10;157(2):329-39. doi: 10.1016/j.cell.2014.02.040.
18. Mok CK(1), Lee HH, Lestra M, Nicholls JM, Chan MC, Sia SF, Zhu H, Poon LL, Guan Y, Peiris JS. Amino acid substitutions in polymerase basic protein 2 gene contribute to the pathogenicity of the novel A/H7N9 influenza virus in mammalian hosts. J Virol. 2014 Mar;88(6):3568-76. doi: 10.1128/JVI.02740- 13.
19. Yamayoshi S(1), Yamada S, Fukuyama S, Murakami S, Zhao D, Uraki R, Watanabe T, Tomita Y, Macken C, Neumann G, Kawaoka Y. Virulence-affecting amino acid changes in the PA protein of H7N9 influenza A viruses. J Virol. 2014 Mar;88(6):3127-34. doi: 10.1128/JVI.03155-13.
20. Zhao Z(1), Yi C, Zhao L, Wang S, Zhou L, Hu Y, Zou W, Chen H, Jin M. PB2-588I enhances 2009 H1N1 pandemic influenza virus virulence by increasing viral replication and exacerbating PB2 inhibition of beta interferon expression. J Virol. 2014 Feb;88(4):2260-7. doi: 10.1128/JVI.03024-13.
21. Huang L(1), Cao Y, Zhou J, Qin K, Zhu W, Zhu Y, Yang L, Wang D, Wei H, Shu Y. A conformational restriction in the influenza A virus neuraminidase binding site by R152 results in a combinational effect of I222T and H274Y on oseltamivir resistance.
22. Pérez-Cidoncha M(1), Killip MJ(2), Asensio VJ(3), Fernández Y(1), Bengoechea JA(4), Randall RE(2), Ortín J(1). Generation of replication-proficient influenza virus NS1 point mutants with interferonhyperinducer phenotype. PLoS One. 2014 Jun 2;9(6):e98668. doi: 10.1371/journal.pone.0098668.
23. Sitaras I(1), Kalthoff D(2), Beer M(2), Peeters B(3), de Jong MC(4). Immune escape mutants of Highly Pathogenic Avian Influenza H5N1 selected using polyclonal sera: identification of key amino acids in the HA protein. PLoS One. 2014 Feb 25;9(2):e84628. doi: 10.1371/journal.pone.0084628.
24. Heaton NS(1), Sachs D, Chen CJ, Hai R, Palese P. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):20248-53. doi: 10.1073/pnas.1320524110.
25. Tamura D(1), Nguyen HT, Sleeman K, Levine M, Mishin VP, Yang H, Guo Z, Okomo-Adhiambo M, Xu X, Stevens J, Gubareva LV. Cell culture-selected substitutions in influenza A(H3N2) neuraminidase affect drug susceptibility assessment. Antimicrob Agents Chemother. 2013 Dec;57(12):6141-6. doi: 10.1128/AAC.01364-13.
26. Kalthoff D(1), Röhrs S, Höper D, Hoffmann B, Bogs J, Stech J, Beer M. Truncation and sequence shuffling of segment 6 generate replication-competent neuraminidase-negative influenza H5N1 viruses. J Virol. 2013 Dec;87(24):13556-68. doi: 10.1128/JVI.02244-13.
27. Zhu X(1), Guo YH, Jiang T, Wang YD, Chan KH, Li XF, Yu W, McBride R, Paulson JC, Yuen KY, Qin CF, Che XY, Wilson IA. A unique and conserved neutralization epitope in H5N1 influenza viruses identified by an antibody against the A/Goose/Guangdong/1/96 hemagglutinin. J Virol. 2013 Dec;87(23):12619-35. doi: 10.1128/JVI.01577-13.
28. Rudneva IA(1), Timofeeva TA, Ignatieva AV, Shilov AA, Krylov PS, Ilyushina NA, Kaverin NV. Pleiotropic effects of hemagglutinin amino acid substitutions of H5 influenza escape mutants. Virology. 2013 Dec;447(1-2):233-9. doi: 10.1016/j.virol.2013.09.013.
29. Lohrmann F(1), Dijkman R, Stertz S, Thiel V, Haller O, Staeheli P, Kochs G. Emergence of a C-terminal seven-amino-acid elongation of NS1 in around 1950 conferred a minor growth advantage to former seasonal influenza A viruses. J Virol. 2013 Oct;87(20):11300-3. doi: 10.1128/JVI.01271-13.
30. Myers JL(1), Wetzel KS, Linderman SL, Li Y, Sullivan CB, Hensley SE. Compensatory hemagglutinin mutations alter antigenic properties of influenza viruses. J Virol. 2013 Oct;87(20):11168-72. doi: 10.1128/JVI.01414-13.
31. Min JY(1), Santos C, Fitch A, Twaddle A, Toyoda Y, DePasse JV, Ghedin E, Subbarao K. Mammalian adaptation in the PB2 gene of avian H5N1 influenza virus. J Virol. 2013. Oct;87(19):10884-8. doi: 10.1128/JVI.01016-13.
32. Long JS(1), Howard WA, Núñez A, Moncorgé O, Lycett S, Banks J, Barclay WS. The effect of the PB2 mutation 627K on highly pathogenic H5N1 avian influenza virus is dependent on the virus lineage. J Virol. 2013 Sep;87(18):9983-96. doi: 10.1128/JVI.01399-13.
33. Roberts KL(1), Leser GP, Ma C, Lamb RA. The amphipathic helix of influenza A virus M2 protein is required for filamentous bud formation and scission of filamentous and spherical particles. J Virol. 2013 Sep;87(18):9973-82. doi: 10.1128/JVI.01363-13.
34. Zaraket H(1), Bridges OA, Duan S, Baranovich T, Yoon SW, Reed ML, Salomon R, Webby RJ, Webster RG, Russell CJ. Increased Acid Stability of the Hemagglutinin Protein Enhances H5N1 Influenza Virus Growth in the Upper Respiratory Tract but Is Insufficient for Transmission in Ferrets. J Virol. 2013 Sep;87(17):9911-22. doi: 10.1128/JVI.01175-13.
35. Li Y(1), Bostick DL, Sullivan CB, Myers JL, Griesemer SB, Stgeorge K, Plotkin JB, Hensley SE. Single hemagglutinin mutations that alter both antigenicity and receptor binding avidity influence influenza virus antigenic clustering. J Virol. 2013 Sep;87(17):9904-10. doi: 10.1128/JVI.01023-13.
- admin
- Site Admin
- Posts: 40066
- Joined: Thu Aug 01, 2013 5:21 am
Who is online
Users browsing this forum: No registered users and 5 guests

