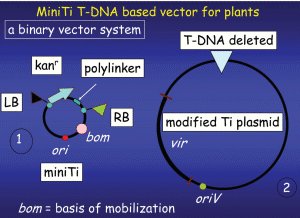
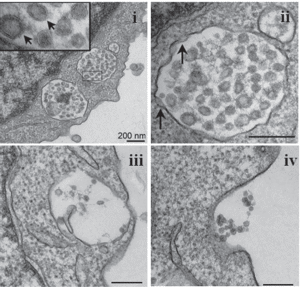
Part 1 of 2
References
Preface1. ISAAA. Pocket K No. 16: Global status of commercialized biotech/GM crops in 2012. Internatioanl Service for the Acquisition of Agri-biotech Application, accessed 14 May 2013,
http://www.isaaa.org/resources/publications/pocketk/16/2. “The inconvenient truth about GM”, Geoffrey Lean, Telegraph, 18 May 2013,
http://www.telegraph.co.uk/earth/agriculture/ geneticmodification/10064255/The-inconvenienttruth- about-GM.html
3. “Monsanto gives up fight for GM plants in Europe” Deutsche Wele, 31 May 2013,
http://www.dw.de/monsantogives- up-fight-for-gm-plants-in-europe/a-16851701
4. “Monsanto to withdraw EU approval requests for new GMO crops”, Charlie Dunmore, Reuters, 17 July 2013, http://
www.reuters.com/article/2013/07/17/us-e ... 6R201307175. “Monsanto shares fall as South Korea joins pause in wheat imports”, Steven Mufson, Wasington Post, 1 June 2013,
http://www.washingtonpost.com/business/economy/ monsanto-shares-fall-as-south-korea-joins-pause-in-wheatimports/ 2013/05/31/5df79a3a-ca2c-11e2-8da7-d274bc611a47_ story.html
6. “US Department of Agriculture probes Oregon Monsanto GM wheat mystery”, Suzanne Goldenberg, Guardian, 22 June 2013,
http://www.guardian.co.uk/environment/2013/ jun/22/agriculture-oregon-monsanto-gm-wheat
7. “Eight European countries ban genetically modified crops, Poland the latest”, 8 January 2013,
http://www.examiner. com/article/eight-european-countries-ban-genetically-modified- crops-poland-the-latest
8. “Genetically modified crops and their significance for sustainable agriculture in Switzerland”, EuropaBio, 22 March 2013,
http://www.europabio.org/news/genetically-modified- crops-and-their-significance-sustainable-agricultureswitzerland
9. “This is the end of GM crops in Denmark”, Niels C. Jensen, DR dk, 29 May 2013,
http://www.dr.dk/Nyheder/Indland/ 2013/05/28/184747.htm (automatically translated by Google).
10. “Italy moves to ban growing of genetically modified maize type”, Reuters, 12 July 2013,
http://uk.reuters.com/article/ 2013/07/12/us-italy-gmo-idUKBRE96B0OS20130712
11. List of GMO-Free Regions, accessed 14 May 2013, http://
www.gmo-free-regions.org/fileadmin/file ... e-regions/ full_list/List_GMO-free_regions_Europe_update_September_ 2010.pdf
12. Ho MW. GM crops facing meltdown in the USA. Science in Society 46, 24-27, 2010.
13. Sirinathsinghji E. US staple crop system failing from GM and monoculture. Science in Society 59 (to appear).
14. “New GMO labelling bill will be the ultimate test between the will of the people versus the greed and power of the biotech industry”, Michelle Goldstein, Natural News, 29 April 2013,
http://www.naturalnews.com/040118_Monsanto_ GMO_labeling_Federal_Bill.html
15. “Green Party calls Monsanto a top risk to health and the environment, urges a moratorium on genetically modified food crops”, Press Release, Green Party, 16 May 2013,
http://www.gp.org/press/pr-national.php?ID=61816. “Peru bans Monsanto and GMOs”, Kristen M, Food Renegad, 3 December 2012,
http://www.foodrenegade.com/17. “Kenya bans the import of all GMOs with immediate effect”, Food Exposed, 21 November 2012,
http://www.foodexposed. co.za/kenya-bans-the-import-of-all-gmos-withimmediate- effect/
18. “Venezuela goes for law against transgenic seeds”, Prensa Latina, 1 June 2013,
http://www.plenglish.com/index. php?option=com_content&task=view&id=1470181&Item id=1
19. “Indefinite moratorium on GM field trials recommended”, Latha Jishnu, Down to Earth, 22 July 2013,
http://www. downtoearth.org.in/content/indefinite-ban-gm-field-trialsrecommended
20. “Mexico – Ground zero in the fight for the future of maize”, Emilio Goday, Inter Press Service, Global issues, 8 May 2013,
http://www.globalissues.org/ news/2013/05/08/16502
21. “The GMO seed cartel”, Ken Roseboro, The Organic & Non- GMO Report, 1 February 2013,
http://www.non-gmoreport. com/articles/february2013/the-gmo-seed-cartel.php
22. Ho MW. Farmer suicides & Bt cotton nightmare unfolding in India. Science in Society 45, 32-39, 2010.
23. Ho MW and Lim LC. The Case for a GM-Free Sustainable World, Independent Science Panel Report, Institute of Science in Society and Third World Network, London and Penang, 2003; republished GM-Free, Exposing the Hazards of Biotechnology to Ensure the Integrity of Our Food Supply, Vitalhealth Publishing, Ridgefield, Ct., 2004 (both available from ISIS online bookstore
http://www.i-sis.org.uk/onlinestore/ books.php#1)
24. Mcintyre BD, Herren HR, Wakhungu J and Watson RT eds. Agriculture at a Crossroads, International Assessment of Agricultural Knowledge, Science and Technology for Development, Synthesis Report, Island Press, Washington D.C., 2009.
http://www.unep.org/dewa/agassessment/reports/ IAASTD/EN/Agriculture%20at%20a%20Crossroads_Synthesis% 20Report%20(English).pdf
25. Ho MW, Burcher S, Lim LC, Cummins J. et al. Food Futures Now, Organic, Sustainable, Fossil Fuel Free, ISIS/TWN, London/ Penang, 2008.
http://www.i-sis.org.uk/foodFutures. php
26. Agriculture at a Crossroads, International Assessment of Agricultural Knowledge, Science and Technology for Development, Synthesis Report, 2009, Island Press, Washington DC,
http://www.unep.org/dewa/agassessment/reports/ IAASTD/EN/Agriculture%20at%20a%20Crossroads_Synthesis% 20Report%20(English).pdf
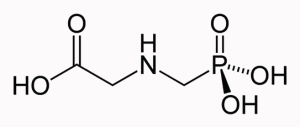
1 Double Jeopardy of Glyphosate & Glyphosate Tolerant Crops1. Séralini G-E, Clair E, Mesnage R, Gress S, Defarge N, Malatesta M, Hennequin D, de Vendômois J-S. Long term toxicity of a Roundup herbicide and a Roundup-tolera,nt genetically modified maize. Food and Chemical Toxicology published or the lonline September 2012.
http://dx.doi.org/10.1016/j. fct.2012.08.005
2. Saunders PT. Excess Cancers and Deaths with GM Feed: the Stats Stand Up. Science in Society 56, 5-6, 2012.
3. “Study linking GM maize to cancer must be taken seriously by regulators”, John Vidal, The Guardian, 28 September 2012,
http://www.guardian.co.uk/environment/2012/sep/28/ study-gm-maize-cancer
4. “Food herbicide residues set to rise as much as 150 times”, Press Release, GM Freeze, 8 February 2012,
http://www. gmfreeze.org/news-releases/180/
5. Environmental Protection Agency. 40 DRF Part 180 [EPA=HQ-P{{-2012-0132; FRL-9384-3] Glyphosate; Pesticide Tolerances.
http://www.regulations.gov/#!submitComm ... -0132-00096. Antoniou M, Habib M, Howard CV, Jennings RC, Leifert C, Nodari RO, Robinson C, Fagan J. Roundup and birth defects: Is the public being kept in the dark? Earth Open Source, 2011.
7. Antoniou M, Habib MEM, Howard CV, Jennings RC, Leifert C, Nodari RO, Robinson CJ and Fagan J. Teratogenic Effects of Glyphosate-Based Herbicides: Divergence of Regulatory Decisions from Scientific Evidence. J Environmental Analytical Toxicology 2012, S4-006, doi: 10.4172/2161-0525.S4-006
8. Sirinathsinghji E and Ho MW. EU regulators Regulators and Monstanto Exposed for Hiding Glyphosate Toxicity. Science in Society 51, 46-48, 2011
9. Benbrook C. Impacts of genetically engineered crops on pesticide use in the US – the first sixteen years. Environmental Sciences Europe 2012, 24, 24 doi:10.1185.2190-4715- 24-24.
10. Sirinathsinghji E. Study confirms GM crops lead to increased pesticide use. Science in Society 56, 8-9, 2012.
11. Benbrook C. GE crop risk assessment challenges: an overview. Food Safety News 6 May 2013,
http://www.foodsafetynews. com/2013/05/ge-crop-risk-assessment-challengesan- overview/
12. WHO (1994) Glyphosate. Environmental health criteria no.159.World Health Organization, Geneva
13. European Commission 2010. Commission Directive 2010/77/ EU of 10 November 2010 in Annex I of certain active substances. Official Journal of the European Union, L 293, 11.11.2010.amending Council Directive 91/414/EEC as regards the expiry dates for inclusion Monsanto, Wikipedia, 16th February 2012
http://en.wikipedia.org/wiki/Monsanto14. Monsanto guilty of chemical poisoning in France, Reuters, 16th February 2012
http://in.reuters.com/article/2012/02/13/ france-pesticides-monsanto-idINDEE81C0FQ20120213
15. Steinrücken HC, Amrhein N The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem Biophys Res Commun, 1980, 94, 1207– 1212
16. Ho MW and Cherry B. Glyphosate tolerant crops bring diseases and death. Science in Society 47.12-15, 2010.
17. Sirinathsinghji E. USDA Scientist Reveals All. Glyphosate Hazards to Crops, Soils, Animals and Consumers. Science in Society 53, 36-39, 2012
18. Perkins PJ, Boermans HJ & Stephenson G.R. Toxicity of glyphosate and triclopyr using the frog embryo teratogenesis assay – Xenopus. Environmental Toxicology and Chemistry 2000, 19, 940-945.
19. Howe CM, Berrill M, Pauli BD, Helbing CC, Werry K, Veldhoen N. Toxicity of glyphosate-based pesticides to four North American frog species. Environmental Toxicology and Chemistry. 2004, 23, 1928-38.
20. Soso AB, Barcellos LJ, Ranzani-Paiva MJ, Kreutz LC, Quevedo RM, Anziliero D, Lima M, Silva LB, Ritter F, Bedin AC, Finco JA. Chronic exposure to sub-lethal concentration of a glyphosate-based herbicide alters hormone profiles and affects reproduction of female Jundiá (Rhamdia quelen). Environmental Toxicology and Pharmacology 2007, 23, 308-13
21. Paganelli A, Gnazzo V, Acosta H, Lopez SL and Carrasco AD. Glyphosate-based herbicides produce teratogenic effects on vertebrates by impairing retinoic acid signalling. Chem Res Toxicol, August 9 2010
http://pubs.acs.org/doi/ abs/10.1021/tx1001749
22. Ho MW. Lab study establishes glyphosate link to birth defects. Science in Society 48, 32-33, 2010.
23. Benítez-Leite S, Macchi MA, and Acosta M. Malformaciones Congenitas asociadas a agrotoxicos. Arch. Pediatr. Urug. 2009, 80, 237 – 247.
24. WHO (World Health Organization). 1994. Glyphosate. Environmental Health Criteria. 159.
http://www.inchem.org/ documents/ehc/ehc/ehc159.htm#SectionNumber:7.3
25. Dallegrave, E, DiGiorgio, F, Mantese, Soares Coelho, R, Drawans Pereira, J, Dalsenter, P, and Langeloh, A. The teratogenic potential of the herbicide glyphosate-Roundup in Wistar rats. Toxicology Letters 2003, 142, 45 – 52.77
26. Romano RM, Romano MA, Bernardi MM, Furtado PV, Oliveira CA. Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Archives of Toxicology 2010, 84, 309-17
27. Romano MA, Romano RM, Santos LD, Wisniewski P, Campos DA, de Souza PB, Viau P, Bernardi MM, Nunes MT, de Oliveira CA. Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Archives of Toxicology 2011, Nov 26. [Epub ahead of print]
28. Ermakova IV. Genetically modified soy leads to the decrease of weight and high mortality of rat pups of the first generation. Preliminary studies. EcosInform 2006, 1, 4-9 (in Russian).
29. Ho MW. GM soya-fed rats: stunted, dead, or sterile. Science in Society 33, 4-6, 2007.
30. ISIS. Science and scientist abused. Letter to Nature Biotechnology. Science in Society 36, 8, 2007.
31. Walsh LP, McCormick C, Martin C, Stocco DM. Roundup inhibits steroidogenesis by disrupting steroidogenic acute regulatory (StAR) protein expression. Environmental Health Perspectives 2000, 108, 769-76.
32. Clair E, Mesnage R, Travert C, Séralini GE. A glyphosate-based herbicide induces necrosis and apoptosis in mature rat testicular cells in vitro, and testosterone decrease at lower levels. Toxicology In Vitro 2011 Dec 19. [Epub ahead of print]
33. Sirinathsinghji EC. Glyphosate Kills Rat Testes Cells. Science in Society 54, 34-36.
34. Sirinathsinghji E. Pesticide Illnesses and GM Soybeans. Ban on Aerial Spraying Demanded in Argentina, Science in Society 53, 2012
35. Richard S, Moslemi S, Sipahutar H, Benachour N, Seralini GE. Differential effects of glyphosate and roundup on human placental cells and aromatase. Environmental Health Perspectives 2005, 113, 716-20.
36. Gasnier C, Dumont C, Benachour N, Clair E, Chagnon MC, Séralini GE. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 2009, 262, 184-91. Epub 2009 Jun 17.
37. Hokanson R, Fudge R, Chowdhary R, Busbee D. Alteration of estrogen-regulated gene expression in human cells induced by the agricultural and horticultural herbicide glyphosate. Hum Exp Toxicol 2007, 26, 747-52.
38. Hardell L, Eriksson M, Nordstrom M. Exposure to pesticides as risk factor for non-Hodgkin's lymphoma and hairy cell leukemia: pooled analysis of two Swedish case-control studies. Leuk Lymphoma 2002, 43, 1043-9
39. De Roos AJ, Zahm SH, Cantor KP, Weisenburger DD, Holmes FF, Burmeister LF, Blair A. Integrative assessment of multiple pesticides as risk factors for non-Hodgkin's lymphoma among men. Occup Environ Med 2003, 60, E11.
40. Eriksson M, Hardell L, Carlberg M, Akerman M. Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. Int J Cancer 2008, 123, 1657-63.
41. De Roos AJ, Blair A, Rusiecki JA, Hoppin JA, Svec M, Dosemeci M, Sandler DP, Alavanja MC. Cancer incidence among glyphosate-exposed pesticide applicators in the Agricultural Health Study. Environ Health Perspect 2005, 113, 49-54.
42. George J, Prasad S, Mahmood Z, Shukla Y. Studies on glyphosate-induced carcinogenicity in mouse skin: a proteomic approach. J Proteomics 2010, 73, 951-64. Epub 2010 Jan 4
43. Paz-y-Miño C, Sánchez ME, Arévalo M, Muñoz MJ, Witte T, De-la- Carrera GO, Leone PE. Evaluation of DNA damage in an Ecuadorian population exposed to glyphosate. Genetics and Molecular Biology 2007, 30, 456-460.
44. Peluso M, Munnia A, Bolognesi C, Parodi S. 32P-postlabeling detection of DNA adducts in mice treated with the herbicide Roundup. Environ Mol Mutagen. 1998, 31, 55-9.
45. Peluso M, Merlo F, Munnia A, Bolognesi C, Puntoni R, Parodi S. (32)P-postlabeling detection of DNA adducts in peripheral white blood cells of greenhouse floriculturists from western Liguria, Italy. Cancer Epidemiol Biomarkers Prev. 1996 5, 361-9.
46. Bolognesi C, Bonatti C, Degan P, Gallerani E, Peluso M, Rabboni R, Roggieri P, Abbondandolo A. Genotoxic Activity of Glyphosate and Its Technical Formulation Roundup. Journal of Agricultural and Food Chemistry 1997, 45, 1957−1962.
47. Koller VJ, Fürhacker M, Nersesyan A, Mišík M, Eisenbauer M, Knasmueller S. Cytotoxic and DNA-damaging properties of glyphosate and Roundup in human-derived buccal epithelial cells. Arch Toxicol 2012 Feb 14. [Epub ahead of print]
48. Sirinathsinghji E. Glyphosate Toxic to Mouth Cells and Damages DNA, Roundup Much Worse, Science in Society 54, 38-39, 2012
49. Marc J, Mulner-Lorillon O, Bellé R. Glyphosate-based pesticides affect cell cycle regulation. Biol Cell 2004, 96, 245-9.
50. Bellé R, Le Bouffant R, Morales J, Cosson B, Cormier P, Mulner-Lorillon O. Sea urchin embryo, DNA-damaged cell cycle checkpoint and the mechanisms initiating cancer development. J Soc Biol 2007, 201, 317-27.
51. Cavas T, Konen S. Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay. Mutagenesis 2007, 22, 263–268.
52. Guilherme S, Gaivão I, Santos MA, Pacheco M. European eel (Anguilla anguilla) genotoxic and pro-oxidant responses following short-term exposure to Roundup--a glyphosate-based herbicide. Mutagenesis 2010, 25, 523-30.
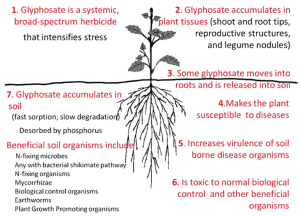
53. Jiraungkoorskul W, Upatham ES, Kruatrachue M, Sahaphong S, Vichasri-Grams S, Pokethitiyook P. Biochemical and histopathological effects of glyphosate herbicide on Nile tilapia (Oreochromis niloticus). Environmental Toxicology 2003, 18, 260-7.
54. Kale PG, Petty BT Jr, Walker S, Ford JB, Dehkordi N, Tarasia S, Tasie BO, Kale R, Sohni YR. Mutagenicity testing of nine herbicides and pesticides currently used in agriculture. Environmental and Molecular Mutagenesis 1995, 25, 148-53.
55. Mañas F, Peralta L, Raviolo J, García Ovando H, Weyers A, Ugnia L, Gonzalez Cid M, Larripa I, Gorla N. Genotoxicity of AMPA, the environmental metabolite of glyphosate, assessed by the Comet assay and cytogenetic tests. Ecotoxicology & Environmental Safety 2009, 72, 834-7.
56. Benachour N and Séralini G-E. Glyphosate formulations Induce Apoptosis and Necrosis in Human Umbilical, Embryonic, and Placental Cells Chem. Res. Toxicol. , 2009, 22, 97–105.
57. Ho MW and Cherry B. Death by multiple poisoning, glyphosate and Roundup. Science in Society 42, 14, 2009.
58. Mesnage R, Bernay B and Séralini G-E. Ethoxylated adjuvants of glyphosate-based herbicids are active principles of human cell toxicity. Toxicology 2012,
http://dx.doi. org/10.1016/j.tox.2012.09.006
59. Barbosa ER, Leiros da Costa MD, Bacheschi LA, Scaff M, Leite CC. Parkinsonism after glycine-derivate exposure. Movement Disorders 2001 16, 565-8.
60. Potrebić O, Jović-Stosić J, Vucinić S, Tadić J, Radulac M. [Acute glyphosate-surfactant poisoning with neurological sequels and fatal outcome]. Vojnosanit Pregl 2009, 66, 758-62.
61. Astiz M, de Alaniz MJ, Marra CA. Antioxidant defense system in rats simultaneously intoxicated with agrochemicals. Environ Toxicol Pharmacol. 2009, 28, 465-73.
62. Astiz M, de Alaniz MJ, Marra CA. Effect of pesticides on cell survival in liver and brain rat tissues. Ecotoxicol Environ Saf 2009, 72, 2025-32. Epub 2009 Jun 2.
63. Ho MW. Cancer a redox disease. Science in Society 54, 12-17, 2012.
64. Gui YX, Fan XN, Wang HM, Wang G, Chen SD. Glyphosate induced cell death through apoptotic and autophagic mechanisms. Neurotoxicology and Teratology 2012, Apr 4. [Epub ahead of print]
65. Garry VF, Harkins ME, Erickson LL, Long-Simpson LK, Holland SE, Burroughs BL. Birth defects, season of conception, and sex of children born to pesticide applicators living in the Red River Valley of Minnesota, USA. Environ Health Perspect 2002, 110 Suppl 3, 441-9.
66. Glusczak L, Miron Ddos S, Moraes BS, Simões RR, Schetinger MR, Morsch VM, Loro VL. Acute effects of glyphosate herbicide on metabolic and enzymatic parameters of silver catfish (Rhamdia quelen). Comp Biochem Physiol C Toxicol Pharmacol. 2007, 146, 519-24.
67. Menéndez-Helman RJ, Ferreyroa GV, dos Santos Afonso M, Salibián A. Glyphosate as an acetylcholinesterase inhibitor in Cnesterodon decemmaculatus. Bull Environ Contam Toxicol 2012, 88, 6-9.
68. Jurewicz J, Hanke W. Prenatal and childhood exposure to pesticides and neurobehavioral development: review of epidemiological studies. Int J Occup Med Environ Health 2008, 21, 121-32.
69. Memorandum on Glyphosate Registration Standard Revision, United States Environment Protection Agency. 1986
http://www.epa.gov/pesticides/chem_sear ... d_reviews/ csr_PC-103601_1-Mar-86_210.pdf
70. The Case for a GM-Free Sustainable World, Independent Science Panel Report, Executive Summary 2003, http://
www.i-sis.org.uk/TheCaseforAGM-FreeSustainableWorld. php
71. Ho MW. Ban glyphosate herbicides now. Science in Society 43, 34-35, 2009.
72. Szarek J, Siwicki A, Andrzejewska A, Terech-Majewska E, Banaszkiewicz T. Effects of the herbicide Roundup on the ultrastructural pattern of hepatocytes in carp (Cyprinus carpio) Mar. Environ. Res 2000, 50, 263–266
73. Malatesta M, Perdoni F, Santin F, Battistelli S, Muller S, Biggiogera F. Hepatoma tissue culture (HTC) cells as a model for investigating the effects of low concentrations of herbicide on cell structure and function. Toxicology In Vitro 2008, 22, 1853–1860
74. Sirinathsinghji E. GM Feed Toxic, Meta-Analysis Reveals. Science in Society 52, 30-32, 2011.
75. Robinson C. Argentina's Roundup human tragedy Science in Society 48, 30, 2010
76. Samsel A and Seneff S. Glyphosate’s suppression of cytochrome P450 enzymes and amino acid biowynthesis by gut microbiome: pathways to modern diseases. Entropy 2013, 15, 1-x manuscripts; doi: 19.3390/e140x000x.
77. Shinabarger DL and Braymer HD. Glyphosate catabolism by Pseudomonas sp. Strain PG2982. J Bacteriol 1986, 168, 702-7.
78. Nie CL, Wang XS, Liu Y, Perrett S and He RQ. Amyloid-like aggregates of neuronal tau induced by formaldehyde promote apoptosis of neuronal cells. BMC Neurosci 2007, 8, 9.
79. Rodloff AC and Krüger M. Chronic Clostridium botulinum infections in farmers. Anaerobe 2012, 18, 226-8.
80. Krüger M. Shehata AA, Schrödl W and Rodloff A. Glyphosate suppresses the antagonistic effect of Enterococcus spp. on Clostridium botulinum. Anaerobe 2013, 20, 74-78.
81. Cherry B. GM crops increase herbicide use in the United States. Science in Society 45 , 44-46, 2010
82. Ho MW. GM crops facing meltdown in the USA. Science in Society 46, 24-27, 2010.
83. WeedScience Database, International Survey of Herbicide Resistant Weeds.
http://www.weedscience.org/In.asp, March 21st 2012.
84. Sirinathsinghji E. Monsanto Defeated By Roundup Resistant Weeds. Science in Society 53, 40-41, 2011.
85. Purdue University, University News Service. “Waterhemp weed showing greater resistance to glyphosate”, 2oth March, 2012
http://www.purdue.edu/newsroom/general/2 011/110929JohnsonWaterhemp.html
86. Bensch CN, Horak MN, Peterson D. Interference of redroot pigweed (Amaranthus retroflexus L.) in green bean (Phaseolus vulgaris L.). Weed Science 51, 37-43. 2003
87. “Glyphosate-resistant weed problem extends to more species, more farms”, Farm Industry News, 29 January 2013,
http://farmindustrynews.com/herbicides/ ... resistant- weed-problem-extends-more-species-more-farms
88. Bernards ML, Crespo RJ, Kruger GR, Gaussoin R,. Tranel PJ. A Waterhemp (Amaranthus tuberculatus) Population Resistant to 2,4-D. Weed Science 2012, 60, 379
89. Report from the 1st National Meeting of Physicians in the Crop-sprayed Towns, Faculty of Medical Sciences, National University of Cordoba, 27th and 28th August 2010 http://
www.reduas.fcm.unc.edu.ar/wp-content/plugins/download- monitor/download.php?id=34
90. Glyphosate-resistant weeds found in multiple Indiana counties. Purdue University News Service, 3rd September 2012
http://www.purdue.edu/newsroom/outreach/2012/120706J ohnsonGlyphosate.html
91. Yamada T. Kremer RJ. De Carmargo e Castro and Wood BW. Glyphosate interactions with physiology, nutrition, and diseases of plants: threats to agricultural sustainability? Europ J Agronomy 2009 31, 111-3.
92. Kremer RJ and Means NE. Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. European Journal of Agronomy 2009, 31, 153-6.
93. Glyphosate, Wikipedia, 4 May 2010,
http://en.wikipedia.org/ wiki/Glyphosate#cite_note-EPAusage-2
94. Zobiole LHS, Oliveira RS Jr, Huber DM, Constantina J, Castro C, Oliveira FA, Oliveira A. Jr. Glyphosate reduces shoot concentrations of mineral nutrients in glyphosate-resistant soybeans. Plant Soil 2010, 328:57-69
95. Cakmak, I, Yazici, A, Tutus, Y, and Ozturk L. Glyphosate reduced seed and leaf concentrations of calcium, magnesium, manganese, and iron in non-glyphosate resistant soybean. European Journal of Agronomy 2009, 31, 114-119.
96. Zobiole LHS, Silvério de Oliveira RS Jr, Kremer RB, Constantina J, Bonatoc CM, Muniz AS. Water use efficiency and photosynthesis of glyphosate-resistant soybean as affected by glyphosate. Pesticide Biochemistry and Physiology 2010, 97, 182-193
97. Zobiole LHS, Bonini EA, Oliveira RS Jr, Kremer RJ, and Ferrarese-Filho O. Glyphosate affects lignin content and amino acid production in glyphosate-resistant soybean. Acta Physiologiae Plantarium 2010, 32, 831-837
98. Fernandes J, Falco WF, Oliveira SL, Caires AR. Changes in chlorophyll a fluorescence of glyphosate-tolerant soybean plants induced by glyphosate: in vivo analysis by laserinduced fluorescence spectroscopy. Applied Optics 2013, 52, 3004-11. doi: 10.1364/AO.52.003004
99. Johal CS & Huber DM. Glyphosate effects on diseases of plants. European Journal of Agronomy 2009 31, 144-152.
100. Neumann G. Risks of Glyphosate Pre-crop Applications inreduced-Tillage Winter Wheat Cropping Systems. Universitat Hohenheim, unpublished. 2012
101. Sirinathsinghji E. GM crops destroyed by US drought but non-GM varieties flourish. Science in Society 56, 6-7, 2012.
102. “Failure to Yield. Evaluating the Performance of Genetically Engineered Crops”. Union of Concerned Scientists. 2009.
http://www.ucsusa.org/assets/documents/ ... riculture/ failure-to-yield.pdf
103. Shi G, Chavas JP, Lauer J. Commercialized transgenic traits, maize productivity and yield risk. Nature Biotechnology 2013, 31,111-4
104. Székács A & Darvas B. Forty Years with Glyphosate. Herbicides - Properties, Synthesis and Control of Weeds, Chapter 14. ISBN: 978-953-307-803-8
105. Tsui MT, Chu LM. Aquatic toxicity of glyphosate-based formulations: comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189-97.
106. Ho MW. Scientists Reveal Glyphosate Poisons Crops and Soil. Science in Society 47, 2010, 12-15
107. Clair E, Linn L, Travert C, Amiel C, Séralini GE, Panoff JM. Effects of Roundup(®) and Glyphosate on Three Food Microorganisms: Geotrichum candidum, Lactococcus lactis subsp. cremoris and Lactobacillus delbrueckii subsp. bulgaricus. Current Microbiology 2012, 64, 486-91.
108. Helander M, Saloniem R and Saikkonen K. Glyphosate in northern ecosystems. Trends in Plant Science 2012, 17, 569- 74.
109. Ho MW. Roundup Kills Frogs. Science in Society 26, 14, 2005.
110. Relyea R A. New effects of Roundup on amphibians: Predators reduce herbicide mortality; herbicides induce antipredator morphology. Ecological Applications 2012, 22, 634–647.
111. Glyphosate: lethal for amphibians in the Basque Country. 2nd February 2012
http://www.basqueresearch.com/berria_ irakurri.asp?Berri_Kod=3735&hizk=I
112. Cuhra M, Traavik T, Bohn T. Clone- and age-dependent toxicity of a glyphosate commercial formulation and its active ingredient in Daphnia magna. Ecotoxicology 2012 Dec 6.[Epub ahead of print]
113. Vera MS, Lagomarsino L, Sylvester M, et al. New evidences of Roundup (glyphosate formulation) impact on the periphyton community and the water quality of freshwater ecosystems. Ecotoxicology 2010, 19, 710-21.
114. Vera MA, Di Fiori E, Lagomarsino L, et al. Direct and indirect effects of the glyphosate formulation Atanor ® on freshwater microbial communities. Ecotoxicology 2012, 21, 1805-16.
115. Ho MW. World water supply in jeopardy. Science in Society 56, 38-43, 2012.
116. Sirinathsinghji E. GM Crops and Water – A Recipe for Disaster. Science in Society 58, to appear.
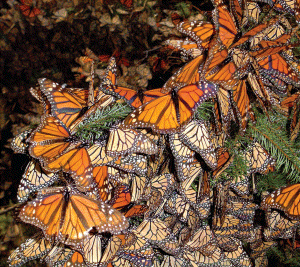
117. Sirinathsinghji E. Glyphosate & Monarch Butterfly Decline. Science in Society 52, 32-33, 2011
118. Pleasants JM & Oberhauser KS. Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conservation and Diversity 2012, doi: 10.1111/j.1752-4598.2012.00196.x
119. McLaren PJ, Cave JG, Parker EM, Slocombe RF. Chondrodysplastic calves in Northeast Victoria. Veterinary Pathology 2007, 44, 342-54
120. Sirinathsinghji E. GM Soy Linked to Illnesses in Farm Pigs. Science in Society 55, 8-9, 2012.
121. Sirinathsinghji E. GM crops and water – a recipe for disaster. Science in Society 58 (in press).
122. Sanchís J, Kantiani L, Llorca M, Rubio F, Ginebreda A, Fraile J, Garrido T, Farré M. Determination of glyphosate in ground water samples using an ultrasensitive immunoassay and confirmation by on-line solid-phase extraction followed by liquid chromatography coupled to tandem mass spectrometry. Analytical and Bioanalytical Chemistry 2012, 402, 2335-45.
123. Chang FC, Simcik MF, Capel PD. Occurrence and fate of the herbicide glyphosate and its degradate aminomethylphosphonic acid in the atmosphere. Environmental Toxicology & Chemistry 2011, 30, 548-55.
124. Herbicide in Urine. Ithaka Journal.
http://www.ithaka-journal. net/herbizide-im-urin , 22nd April 2012.
125. Bill would ban pesticides and reassess the use of glyphosate. GMWatch, 20th September 2012,
http://www. gmwatch.org/latest-listing/51-2012/14210-bill-would-banpesticides- and-reassess-the-use-of-glyphosate-in-brazil
126. Ho MW, Burcher S, Lim LC, et al. Food Futures Now *Organic*Sustainable*Fossil Fuel Free, ISIS/TWN, London/ Penang, 2008.
http://www.i-sis.org.uk/foodFutures.php2 Bt Crops Failing & Harmful to Health and Environment1. Benbrook C. Impacts of genetically engineered crops on pesticide use in the U.S. -the first sixteen years. Environmental Sciences Europe 2012, 24,24 doi:10.1186/2190-4715- 24-24
2. Ho MW and Saunders PT. Transgenic cotton offers no advantage. Science in Society 38, 30, 2008.
3. Bt failure to hit cotton yield by 40%: Govt. Dnaindia.com, 26th November 2012
http://www.dnaindia.com/mumbai/ report_bt-failure-to-hit-cotton-yield-by-40pct-govt_1769428
4. Maharashtra reports Bt cotton failure in 4 Million Hector – Thousands Cotton Farmers to Council Hall Nagpur on 11th December for compensation.
http://vidarbhatimes. blogspot.co.uk/
5. Ministry blames Bt cotton for farmer suicides. hindustantimes. com, 26th March 2012
http://www.hindustantimes. com/News-Feed/Business/Ministry-blames-Bt-cotton-forfarmer- suicides/Article1-830798.aspx
6. Ho MW. Farmer suicides and Bt cotton nightmare unfolding in India. Science in Society 45, 32-39, 2010.
7. “Pesticides make a comeback”, Ian Berry, The Wall Street Journal, 21 May 2013,
http://online.wsj.com/article/SB100014 24127887323463704578496923254944066.html
8. Cummins J. Bt toxins in Genetically Modified Crops : Regulation by Deceit. Science in Society 22, 32, 2004.
9. Gurian-Sherman D. Failure to yield: Evaluating the performance of genetically engineered crops. Union of Concerned Scientists. 2009.
http://www.ucsusa.org/assets/ documents/food_and_agriculture/failure-to-yield.pdf
10. Shi G, Chavas JP, Lauer J. Commercialized transgenic traits, maize productivity and yield risk. Nature Biotechnology 2013, 31,111-4
11. Benbrook C. GE crop risk assessment challenges: an overview. Food Safety News 6 May 2013,
http://www.foodsafetynews. com/2013/05/ge-crop-risk-assessment-challengesan- overview/
12. Séralini G-E, Mesnage R, Clair E, Gress S, Vendômois J, Cellier D. Genetically modified crops safety assessments: present limits and possible improvements. Environmental Sciences Europe 2011, 23, 10-20
13. Sirinathsinghji E. GM feed toxic, new meta-analysis confirms. Science in Society 52, 30-32, 2011
14. Mesnage R,Clair E, Gress S,Then C, Székács A,Séralini G-E. Cytotoxicity on human cells of Cry1Ab and Cry1Ac Bt insecticidal toxins alone or with a glyphosate-based herbicide. DOI 10.1002/jat.2712
15. Sirinathsinghji E. Bt Toxin Kills Human Kidney Cells. Science in Society 54, 36-38, 2012
16. Vázquez-Padrón RI, Gonzáles-Cabrera J, García-Tovar C, Neri-Bazan L, Lopéz-Revilla R, Hernández M, Moreno-Fierro L, de la Riva GA. Cry1Ac protoxin from Bacillus thuringiensis sp. kurstaki HD73 binds to surface proteins in the mouse small intestine. Biochemical Biophysical Research Communications 2010, 271, 54-8.
17. Finamore A, Roselli M, Britti S, Monastra G, Ambra R, Turrini A, Mengheri E. Intestinal and peripheral immune response to MON810 maize ingestion in weaning and old mice. Journal of Agricultural and Food Chemistry 2008, 56, 11533-9.
18. Mezzomo BP, Miranda-Vilela AL, Freire IdS, Barbosa LC, Portilho FA, Lacava ZG, Grisolia CK. Effects of oral administration of Bacillus thuringiensis as spore-crystal strains Cry1Aa, Cry1Ab, Cry1Ac or Cry2Aa on hematologic and genotoxic endpoints of Swiss albino mice. Journal of Hematology Thromboembolic Diseases 2013, 1,104. doi: 10.4172/ jhtd.1000104
19. Ho MW. More illnesses linked to Bt crops. Science in Society 30, 8-10, 2006.
20. Aris A, Leblanc S. Maternal and fetal exposure to pesticides associated to genetically modified foods in Eastern Townships of Quebec, Canada. Reproductive Toxicolology, 2011,31, 528-33
21. Ho MW. GM maize reduces fertility & deregulates genes in mice. Science in Society 41, 40-41, 2009.
22. Kranthi KR, Naidu S, Dhawad CS, Tatwawadi A, Mate K, Patil E, Bharose AA,. Behere GT, Wadaskar RM and Kranthi S. Temporal and intra-plant variability of Cry1Ac expression in Bt-cotton and its influence on the survival of the cotton bollworm, Helicoverpa armigera (Hübner) (Noctuidae: Lepidoptera). Current Science 2005, 89, 291-7.
23. Wan P, Zhang Y, Wu K, Huang M. Seasonal expression profiles of insecticidal protein and control efficacy against Helicoverpa armigera for Bt cotton in the Yangtze River valley of China. Journal of Economic Entomology 2005, 98, 195-201.
24. “The GMO Emperor has no Clothes” Navdanya International report, 2011
http://www.navdanyainternational.it/images/ doc/Full_Report_Rapporto_completo.pdf
25. Gala R. Organic cotton beats Bt cotton. Science in Society 27, 49-50, 2005.