Nature Biotechnology 25, 1351 - 1354 (2007)
doi:10.1038/nbt1207-1351
NOTICE: THIS WORK MAY BE PROTECTED BY COPYRIGHT
YOU ARE REQUIRED TO READ THE COPYRIGHT NOTICE AT THIS LINK BEFORE YOU READ THE FOLLOWING WORK, THAT IS AVAILABLE SOLELY FOR PRIVATE STUDY, SCHOLARSHIP OR RESEARCH PURSUANT TO 17 U.S.C. SECTION 107 AND 108. IN THE EVENT THAT THE LIBRARY DETERMINES THAT UNLAWFUL COPYING OF THIS WORK HAS OCCURRED, THE LIBRARY HAS THE RIGHT TO BLOCK THE I.P. ADDRESS AT WHICH THE UNLAWFUL COPYING APPEARED TO HAVE OCCURRED. THANK YOU FOR RESPECTING THE RIGHTS OF COPYRIGHT OWNERS.
GM soybeans—revisiting a controversial format
Irina V. Ermakova1
Institute of Higher Nervous Activity and Neurophysiology, Russian Academy of Sciences, Butlerov str., 5a, Moscow, Russia. e-mail: [email protected]
To the editor:
I was grateful to you for inviting me to discuss some of my experimental results in Nature Biotechnology; however, the Feature entitled “[Genetically modified] GM soybeans and health safety—a controversy reexamined,” as published in the September issue1, presents a flawed picture of my work. Although I thank Bruce Chassy, Val Giddings, Vivian Moses and Alan McHughen (Chassy et al.) for their detailed analysis of my work, remarks and recommendations, I am concerned that your readers will be misled by several of their comments. I also would like to clarify some issues concerning the manner in which this article was commissioned, a process that raises questions about editorial standards and practice at your journal. In my comments below, I first address the questions raised about my experiments and findings in the order in which they were raised in the Feature. I then raise some general concerns about the commissioning, proofing and production process. And, finally, I outline my responses to the criticism in the Feature of my research.
On p. 981, Chassy et al. remark that it is “not possible” for me to have obtained Roundup Ready (RR) line 40.3.2 soybeans from the Netherlands supplier of Archer Daniels Midland (ADM; Decatur, IL, USA), adding “the best that can be said is that commercial products sold by ADM would have been an indeterminate and variable mixture of conventional and non-GM soybeans.” On the next page, they assert that I “provided no PCR evidence that the Arcon SJ product did not contain the CP4 5 EPSPS [enolpyruvyl shikimate-3-phosphate synthase] gene or the CP4 EPSPS protein it encodes. These assays are necessary to demonstrate that this control is in fact a non-GM-containing material.” I can only state that my laboratory did receive soy clearly labeled as GM and non-GM soy. Quantitative analysis of RR soy using the 'CP4-LEC-RT-PCR' construct confirmed the presence of this transgene in 100% of the GM soy flour. In the traditional, non-GM soy flour, only traces (0.08 ± 0.04%) of the same construct were present. In fact, we checked all kinds of soy. The analysis of GM soy and non-GM soy was performed by 'blinded' operators (see Fig. 1).
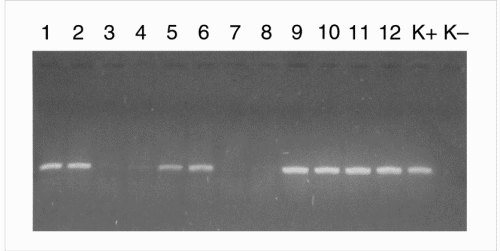
Figure 1: The 'blind' analysis of GM-soy and non-GM-soy samples using PCR.
Lanes 1 and 2, GM-soy (flour); lanes 3 and 4, traditional soy flour; lanes 5 and 6, GM soy protein flour; lanes 7 and 8, traditional soy seeds; lanes 9 and 10, GM soy seeds after temperature treatment (t°); lanes 11 and 12, GM soy seeds; K+, positive control; K−, negative control.
Chassy et al. also note, “Ermakova states that males were not exposed to soy; however, they were placed into cages with females to which soy was provided every day. Consumption of soy by males would have also reduced the ration of soy available to the females.” The last supposition is incorrect. Although males did receive soy during mating—potentially competing for soy rations with females—during this period, the experimental diets of the females were also supplemented with extra soy to correct for any consumption by males. We also performed further investigations where both females and males received soy before and during mating. They state, “after 3 days, the males were moved to the cage of another female where they remained for three additional days.” Again this is incorrect. Males were moved to their own cages after 3 days of mating; they were not moved to the cage of another female because we were going to use pups from different parents to obtain the next generation.
Later on the same page, Chassy et al. write, “Ermakova states that in five trials a total of 100 animals have been studied, which translates to an average of 20 animals per study and ~5 for each experimental group.” Chassy et al. also go on to criticize my study for having too few animals and cite as correct a study by Brake and Evenson2. I was very surprised by these remarks, because they are wrong. We studied 100 adult animals and 396 pups. To obtain the first generation in the main series of experiments, we used 9 females and 6 males (3 females crossed with 2 males in turn) in the control, GM-soy-fed and traditional-soy-fed groups. To clarify matters, I would like to add Table 1, which is similar, but not the same as Table 2 originally supplied by me and printed in the September Feature. In some cases, females didn't give birth; however, the reason for this can be clarified only after investigation of many more females and males. In addition a large number of pups (up to 89) were studied in each of these groups (Table 1). To obtain the second generation, we mated 12 females and 12 males (3 females crossed with 3 males in turn). The research of Brake and Evenson differs from my work in that they used fewer animals for breeding and investigation in their feeding study. In addition, for each diet (transgenic or conventional soybean) in their multigenerational mouse study, they used the following breeding scheme: two females and two males were used to obtain the first generation; six females and three males were then used for each subsequent generation. Also Brake and Evenson studied many fewer pups in each group than we did in our experiments.

Table 1 - Comparison of different kinds of chow on rat pup mortalitya
In discussing the number of animals studied and the pooling of results, Chassy et al. are also concerned that “... it is not standard practice to pool data from” different studies. Again, I disagree. In human studies, it is now, in fact, regarded as good practice to pool randomized, controlled, clinical studies across trials to get a better picture of the effect of the treatment on health and disease. My design merely substitutes rats for humans.
At the bottom of p. 982, Chassy et al. remark that “it is also not stated whether the litters were balanced with regard to number of pups and gender.” The birth rate was similar in all groups: on average 10–11 pups per female (no significant difference). There were also no significant differences in body weights of males and females in all groups 2 weeks after birth. The data are as follows in the two series: in the control group (males, 30.2 ± 1.6; females, 30.7 ± 1.2); in the group receiving traditional soy (males, 27.0 ± 0.9; females, 26.3 ± 1.3); in the group receiving GM soy (males, 26.8 ± 2.0; females, 25.8 ± 1.6); and in the group receiving protein isolate GM soy (males, 27.1 ± 0.8; females, 26.3 ± 1.0). Similar data were obtained in other experiments.
When discussing my experimental design for the study, Chassy et al. comment that the 2-week timing for weight measurement of the animals makes “comparison with literature values difficult.” Specifically, they say, “Parental animals should be weighed on the first day of dosing and each week after. Parental females should be weighed at a minimum on gestation days 0, 7, 14 and 21 and during lactation on the same days as the weighing of the pups. Pups should be weighed individually at birth, or soon thereafter, and on days 4, 7, 14 and 21 of lactation. Ermakova reports the weight of pups at 2 weeks of age.” To clarify matters, the experimental design was as follows: we weighed males and females before mating, and then weighed males every week. We didn't weigh pregnant females because they had different numbers of embryos, which would have influenced their weights. We didn't want to touch pups and disturb their mothers and therefore didn't weigh pups during the first 2 weeks; females could have discarded pups if they were handled. Therefore, all pups were weighed 2 weeks after birth and most of them again 1 and 2 months after the birth.
My response to the remark that “no information is provided about external variables that can affect behavior, such as sound level, temperature, humidity, lighting, odors, time of day and environmental distractions” is that I could have provided this information if I had been asked: the cages of GM-fed and non-GM-fed animals were kept in the same room, so variables such as sound level, temperature, humidity, lighting, odors, time of day and environmental distractions would have been exactly the same between cages. Thus, the differences in health between the GM-fed and non-GM-fed groups observed in my study could not be attributable to external variables.
On p. 983, Chassy et al. comment “no actual data from behavioral studies are presented.” The main focus of my research was to study the physiological state of rats, and then the effect of GM soy on their behavior. I believe that high mortality of pups, the small weights of some surviving pups and the absence of a second generation were the most important and disturbing results of my work. I feel that the data of my behavioral experiments, which I describe briefly below, could be the subject of a separate paper. There were very slight differences between groups in the open field (a standardized environmental arrangement for studying emotionality, spontaneous exploratory activity and locomotor activity). Even so, anxiety in the 'light-dark' test was higher in females, males and offspring receiving GM soy than in rats from other groups. Observed differences in behavior between the sexes of adult animals and also pups were found in this test. Males from groups fed GM soy had low horizontal and vertical activity, a small number of transitions and spent more time in the dark box than males from other groups. The same was true for the male pups. In contrast, females from groups fed GM soy and female pups from GM-soy groups were more active and restless, spent more time at the lit box and had more transitions than females from other groups. It was quite interesting that the pups displayed the same gender-related behavioral differences as adult animals. It is possible that the sex effect could be connected with the higher level of phytoestrogens in GM soy than conventional soy, according to the literature3, 4. This suggestion is being verified by another research group. Preliminary studies in my laboratory to investigate the learning and memory of pups using a modified 'three-panel runway apparatus' indicate an impairment of learning in some tasks of pups from groups fed GM soy.
In discussing my results, Chassy et al. state “Previous reports in the literature have shown no effects of [Roundup Ready] RR soy on birth weights or pup mortality; they have also not shown any effects of RR soy on the testis or in the livers of male rats fed RR soy”2, 5, 6. Later, they concluded that the likely “explanation for the observed health effects [of GM soy] is poor experimental design and conduct as demonstrated by the exceptionally high mortality observed in the controls.” It is necessary to emphasize that studies by these previous investigators had a different aim from my studies and thus they are not comparable. The mortality of pups depends on the feeding protocol and these previous investigators used a different protocol. Chassy et al. also neglect to mention studies that have shown adverse effects of RR soy on testes and livers7, 8, 9.
One of the common criticisms of toxicology studies attempting to assess the influence of GM products on animals is that investigations are performed under unnatural, laboratory conditions. My team tried to avoid this mistake by keeping as close to natural conditions as we could. It is known that in nature the pups have a mortality rate of ~10%. The mortality in our investigations was 8% in the control group (6 pups died out of 72 pups) and 10% in the group fed traditional soy, which is normal for animals in nature. As to their comment that I neglected to report pup mortality at days 0, 1 and 21 and failed to note “the timing and causes of death,” I refer Chassy et al. to my published paper10, which provides the times of pup deaths. We don't yet know the causes of pup death. To accomplish that it will be necessary to perform further biochemical, morphological and genetic studies.
In relation to my mating results, Chassy et al. draw the readers' attention to the Brake and Evanson2 study, which “found no reproductive effects in mice in a multigenerational feeding study with RR soy.” But this is an invalid comparison. In the experiments of Brake and Evenson2 “pregnant mice were fed a transgenic soybean or a non-transgenic (conventional) diet through gestation and lactation....Multigenerational studies were conducted in the same manner.” Thus, the feeding regime for GM soy was completely different from the one used in my experiments, in which rats were offered a GM-soy diet 2 weeks before mating. In the Brake and Evenson experiments, EPSPS gene sequences could influence only embryonic cells in the womb; they could not affect sexual cells and/or organs before and during mating. In contrast, in my experiments, EPSPS gene sequences in GM soy would have had the chance to affect reproductive structures. Thus, my interpretation of these results is that the EPSPS gene sequences ingested by these animals can penetrate and affect rat sexual cells and/or organs11.
I would also like to point out that Chassy et al. misquote me as describing the study by the UK's Advisory Committee of Novel Foods and Process as “funny.” To the contrary, I actually said this was the most serious critique of my work.
At the top of p. 985, Chassy et al. also make the assumption that in Table 5 of the original Feature, the average litter size is six pups and note that the “Wistar rat has a typical litter size of approximately 12.” Again Chassy et al. have misinterpreted my experiments. The litter size was eight pups, not six. This is because 25% of the females from the group receiving GM soy didn't give birth, which was clearly indicated in my response to the question “How were behavior and fertility affected?” I wrote, “The number of pups per female was fewer than in the other groups (8 pups per female instead of 10–11 pups per female) and 25% of females didn't deliver pups at all. These results indicate that GM soy had a deleterious effect on the reproductive function especially of F1 males, but also female rats.”
Many of the above errors could have been clarified—had I been afforded the opportunity to respond. But the publication process for this article gave me no option to do so. I was not given the comments from Chassy et al. to read and respond to before publication. This meant that they spent much of their time raising questions about my work that could have been answered in a full paper. In my view, many of the inaccuracies and criticisms could have been avoided if Chassy et al. had been able to review a full scientific paper from me, rather than my responses to a limited set of questions. The scientific paper would have contained much more information.
I also have several serious concerns about other parts of the editorial process.
First, in e-mail exchanges between us, you refused to publish the whole text of my paper and moreover, when I submitted a paper containing new unpublished data to the journal, it was refused on the grounds that such a paper would be better published elsewhere. Yet, at the same time, Nature Biotechnology found it quite acceptable to assemble and publish a Feature which consisted of a brutal attack on my results.
Second, the galley proof, sent to me by the journal as a 'publication proof' had my name as the author and was vastly different from the article that appeared in print, omitting the introduction by you and the critiques from Chassy et al.
Third, the comments solicited were solely from researchers who I would regard as pro-GM, or with connections to the GM industry, who would likely be hostile to my work. Why were no comments solicited from scientists that have concerns about GMOs [genetically modified organisms]? The process and article were therefore not objective. Many independent scientists were unhappy with the format and their understanding of the commissioning process, and indeed sent me letters stating so.
And fourth, on the proof, many of my references in the original draft had also been removed. In the final published article, the comments of the pro-GM group included many references, potentially distorting the perception of my work as inferior and unsupported by the literature in comparison to the critiques.
I now turn to the critical comments concerning the publication of my research.
Chassy et al. ask if I “had external funding, why are we not told who provided such significant funding?” I could easily have provided this information, if it had been requested of me—but it wasn't. To clarify, I started experiments as an addition to my existing work and then included them as part of my regular research. Because I couldn't find evidence in the scientific literature of the effect of GMOs on the behavior of animals and their offspring, I decided to begin my own experiments. I also planned to try to use special GMOs to improve memory and learning in rats and for treating animals with diseases (such as epilepsy, Parkinson's disease and others). For my investigations, I used material and equipment at my institute, my own salary and a small amount of personal funds.
They also criticize me for failing to publish my work in the peer-reviewed literature and for widely publicizing my work at various congresses, meetings, press conferences and on the internet without providing sufficient experimental support for my claims. I would respond that I have already sent papers into peer-reviewed journals (one paper was submitted a year ago). And what they fail to acknowledge is the difficulty that I have encountered in publishing this work in the peer-reviewed literature—perhaps reflecting the reluctance of the predominantly industry-funded agbiotech community to condone the publication of studies that detail negative effects of GMOs. I am not against GMOs, but wish to promote more safe and effective approaches as much as I can.
When I started these experiments, I didn't expect that the work would attract so much interest. I only thought that scientists would repeat my experiments and confirm or refute my results. The wide interest in my work has not been confined only to investigations of the safety of GMOs, but has extended also to its implications for DNA and gene transfer, ecology and so on. I never sought out journalists. Every attack on me and my work by those allied to the biotech industry or by members of the media has served to create more interest from journalists, scientists, physicians and ecologists. After Nature Biotechnology published the criticism of my work, I have received even more requests to give interviews and invitations to participate at different conferences and meetings.
I would add that I concur with Chassy et al. in that “science needs to be repeated and to stand the test of time.” Most peer-reviewed articles containing evidence of negative effects of GMOs have been criticized and suppressed in much the same way as my own research. I feel this is because there is pressure to dismiss such studies because a huge amount of money has been invested in GMOs. All I have tried to do is to provide evidence of a potential problem with the safety of GM soy.
In 2005, I was concerned when I found the adverse effects of GM soy on rats and their offspring, particularly as the soy I used (Roundup Ready line 40.3.2) is widely eaten by people. I therefore appealed to the international scientific community to repeat my experiments with this GM soy and to extend such studies of other GM plants. In the ensuing 2 years, nobody has repeated this research completely, even though these experiments are easily repeated. However, I am not alone in identifying the adverse health and safety effects of GM products. The scientific literature also details the adverse effects of GM crops on insects12, 13, 14 and mammals7, 8, 9, 15, 16, 17, as well as the presence of foreign DNA in the cells of adult animals and their offspring that have been fed a GMO diet11, 18, 19, 20, 21, 22, 23. Russian researchers performed similar experiments with protein-isolate of GM soy (RR, 40.3.2), showing negative influence of it on mice offspring24. I agree with those scientists of the opinion that these adverse effects could be imperfections in gene transformation methods25, 26, 27. I believe that it is possible to improve these methods, to make them absolutely safe for humans and the environment. Consequently, the adverse effects of GMOs demonstrated in my experiments deserve further investigation. Experiments like mine can only help to inform the biotech community of possible problems with their products that they may not be aware of so solutions can be found. In this context, I would be very grateful to receive samples of transgenic products from companies or other laboratories around the world for my ongoing investigations using rats.
References
1. Marshall, A. Nat. Biotechnol. 25, 981–987 (2007). | Article | PubMed | ChemPort |
2. Brake, D.G. & Evenson, D.P. Food Chem. Toxicol. 42, 29–36 (2004). | Article | PubMed | ISI | ChemPort |
3. McCue, P. & Shetty, K. Crit. Rev. Food Sci. Nutr. 44, 361–367 (2004). | Article | PubMed | ChemPort |
4. Wuttke, W., Jarry, H. & Seidlová-Wuttke, D. Ageing Res. Rev. 6, 150–188 (2007). | Article | PubMed | ChemPort |
5. Teshima, R. et al. J. Food Hyg. Soc. Japan. 41, 188–193 (2000). | Article | ISI | ChemPort |
6. Zhu, Y., Li, D., Wang, F., Yin, J. & Jin, H. Arch. Anim. Nutr. 58, 295–310 (2004). | Article | PubMed | ISI | ChemPort |
7. Malatesta, M. et al. Cell Struct. Funct. 27, 173–180 (2002). | Article | PubMed |
8. Malatesta, M. et al. Eur. J. Histochem., 47, 385–388 (2003). | PubMed | ChemPort |
9. Vecchio, L., Cisterna, B., Malatesta, M., Martin, T.E. & Biggiogera, B. Eur. J. Histochem. 48, 449–453 (2003).
10. Ermakova, I. in Proceedings 'Epigenetics, Transgenic Plants and Risk Assessment',December 1, 2005 Literaturhaus, Frankfurt am Main, Germany, 41–48 (Institute for Applied Ecology, Freiberg, Germany 2006) <http://www.oeko.de/oekodoc/277/2006-002-en.pdf>
11. Schubbert, R., Hohlweg, U., Renz, D. & Doerfler, W. Molec. Genes Genet. 259, 569–576 (1998). | ChemPort |
12. Birch, A.N.E., Geoghegan, I.E., Majerus, M.E.N., McNicol, J.W., Hackett, C.A., Gatehouse, A.M.R. & Gatehouse, J.A. Mol. Breeding 5, 75–83 (1999). | Article | ISI |
13. Losey, J.E., Rayor, L.S. & Carter, M.E. Nature 399, 214 (1999). | Article | PubMed | ISI | ChemPort |
14. Zangerl, R. et al. Proc. Natl. Acad. Sci. USA. 98, 11908–11912 (2001). | Article | PubMed | ChemPort |
15. Pusztai, A. & Bardocz, S. in Biology of Nutrition in Growing Animals (eds. Mosenthin, R., Zentek, J. & Zebrowska, T.) 513–540 (Elsevier, New York, 2006).
16. Ewen, S.W. & Pusztai, A. Lancet 354, 9187 (1999).
17. Prescott, V.E. et al. J. Agr. Food Chem. 53, 9023–9030 (2005). | Article | ChemPort |
18. Schubbert, R., Lettmann, C. & Doerfler, W. Mol. Gen. Genet. 242, 495–504 (1994). | Article | PubMed | ISI | ChemPort |
19. Doerfler, W. Curr. Top. Microbiol. Immunol. 197, 209–224 (1995). | PubMed | ChemPort |
20. Doerfler, W. Adv. Cancer Res. 66, 313–344 (1995). | PubMed | ChemPort |
21. Mercer, D.K., Scott, K.P., Bruce-Johnson, W.A., Glover, L.A. & Flint, H.J. Appl. Env. Microbiol. 65, 6–10 (1999). | ChemPort |
22. Netherwood, T. et al. J. Appl. Environ. Microbiol. 65, 11, 5139–5141 (1999).
23. Chowdhury, E.H. et al. Vet. Hum. Toxicol. 45, 95–96 (2003). | PubMed | ChemPort |
24. Konovalova, M.A. & Blinov, V.A. Second All-Russian Symposium 'Transgenic Plants and Biosafety, Moscow, October 22–25, 2007, (ed. Kuznetsov, V.V.) 22–25 (K.A. Timiryazev Institute of Plant Physiology, Russian Academy of Sciences, Moscow,2007).
25. Ho, M.-W. & Tappeser, B. in Transboundary Movement of Living Modified Organisms Resulting from Modern Biotechnology: Issues and Opportunities for Policy-Makers (Mulongoy, K.J. ed.) 171–193 (International Academy of the Environment, Switzerland, 1997).
26. Kuznetsov, V.V. & Kulikov A.M. Russian Chem. J. 69, 4, 70–83 (2005).
27. Wilson, A., Latham, J. & Steinbrecher, R. Biotechnol. Genet. Eng. Rev. 23, 209–237 (2006). | ChemPort |
